Biofilms in Endodontics-Current Status and Future Directions
- PMID: 28800075
- PMCID: PMC5578138
- DOI: 10.3390/ijms18081748
Biofilms in Endodontics-Current Status and Future Directions
Abstract
Microbiota are found in highly organized and complex entities, known as biofilms, the characteristics of which are fundamentally different from microbes in planktonic suspensions. Root canal infections are biofilm mediated. The complexity and variability of the root canal system, together with the multi-species nature of biofilms, make disinfection of this system extremely challenging. Microbial persistence appears to be the most important factor for failure of root canal treatment and this could further have an impact on pain and quality of life. Biofilm removal is accomplished by a chemo-mechanical process, using specific instruments and disinfecting chemicals in the form of irrigants and/or intracanal medicaments. Endodontic research has focused on the characterization of root canal biofilms and the clinical methods to disrupt the biofilms in addition to achieving microbial killing. In this narrative review, we discuss the role of microbial biofilms in endodontics and review the literature on the role of root canal disinfectants and disinfectant-activating methods on biofilm removal.
Keywords: bacteria; disinfection; extracellular polysaccharide; irrigation; review; root canal.
Conflict of interest statement
The authors declare no conflict of interest.
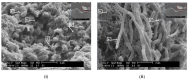
Figures



References
-
- Diaz P.I. Microbial diversity and interactions in subgingival biofilm communities. Front. Oral Biol. 2012;15:17–40. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

