Simultaneous assessment of blood coagulation and hematocrit levels in dielectric blood coagulometry
- PMID: 28800301
- PMCID: PMC5676769
- DOI: 10.3233/BIR-16118
Simultaneous assessment of blood coagulation and hematocrit levels in dielectric blood coagulometry
Abstract
Background: In a whole blood coagulation test, the concentration of any in vitro diagnostic agent in plasma is dependent on the hematocrit level but its impact on the test result is unknown.
Objective: The aim of this work was to clarify the effects of reagent concentration, particularly Ca2+, and to find a method for hematocrit estimation compatible with the coagulation test.
Methods: Whole blood coagulation tests by dielectric blood coagulometry (DBCM) and rotational thromboelastometry were performed with various concentrations of Ca2+ or on samples with different hematocrit levels. DBCM data from a previous clinical study of patients who underwent total knee arthroplasty were re-analyzed.
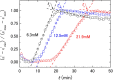
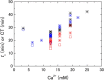
Results: Clear Ca2+ concentration and hematocrit level dependences of the characteristic times of blood coagulation were observed. Rouleau formation made hematocrit estimation difficult in DBCM, but use of permittivity at around 3 MHz made it possible. The re-analyzed clinical data showed a good correlation between permittivity at 3 MHz and hematocrit level (R2=0.83).
Conclusions: Changes in the hematocrit level may affect whole blood coagulation tests. DBCM has the potential to overcome this effect with some automated correction using results from simultaneous evaluations of the hematocrit level and blood coagulability.
Keywords: Dielectric spectroscopy; interfacial polarization; rotational thromboelastometry; rouleau formation; whole blood coagulation test.
Conflict of interest statement
The authors declare the following competing financial interests: Y.H., M.-A.B., K.M., S.L., A.M. and S.O. are employees of Sony Corp. The other authors declare no competing financial interests.
Figures





References
-
- Chiba S, Uchibori K, Fujiwara T, Ogata T, Yamauchi S, Shirai T, et al.. Dielectric blood coagulometry as a novel coagulation test. J Sci Res Rep. 2015;4:180–186.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

