Adding abiraterone to androgen deprivation therapy in men with metastatic hormone-sensitive prostate cancer: A systematic review and meta-analysis
- PMID: 28800492
- PMCID: PMC5630199
- DOI: 10.1016/j.ejca.2017.07.003
Adding abiraterone to androgen deprivation therapy in men with metastatic hormone-sensitive prostate cancer: A systematic review and meta-analysis
Abstract
Background: There is a need to synthesise the results of numerous randomised controlled trials evaluating the addition of therapies to androgen deprivation therapy (ADT) for men with metastatic hormone-sensitive prostate cancer (mHSPC). This systematic review aims to assess the effects of adding abiraterone acetate plus prednisone/prednisolone (AAP) to ADT.
Methods: Using our framework for adaptive meta-analysis (FAME), we started the review process before trials had been reported and worked collaboratively with trial investigators to anticipate when eligible trial results would emerge. Thus, we could determine the earliest opportunity for reliable meta-analysis and take account of unavailable trials in interpreting results. We searched multiple sources for trials comparing AAP plus ADT versus ADT in men with mHSPC. We obtained results for the primary outcome of overall survival (OS), secondary outcomes of clinical/radiological progression-free survival (PFS) and grade III-IV and grade V toxicity direct from trial teams. Hazard ratios (HRs) for the effects of AAP plus ADT on OS and PFS, Peto Odds Ratios (Peto ORs) for the effects on acute toxicity and interaction HRs for the effects on OS by patient subgroups were combined across trials using fixed-effect meta-analysis.
Findings: We identified three eligible trials, one of which was still recruiting (PEACE-1 (NCT01957436)). Results from the two remaining trials (LATITUDE (NCT01715285) and STAMPEDE (NCT00268476)), representing 82% of all men randomised to AAP plus ADT versus ADT (without docetaxel in either arm), showed a highly significant 38% reduction in the risk of death with AAP plus ADT (HR = 0.62, 95% confidence interval [CI] = 0.53-0.71, p = 0.55 × 10-10), that translates into a 14% absolute improvement in 3-year OS. Despite differences in PFS definitions across trials, we also observed a consistent and highly significant 55% reduction in the risk of clinical/radiological PFS (HR = 0.45, 95% CI = 0.40-0.51, p = 0.66 × 10-36) with the addition of AAP, that translates to a 28% absolute improvement at 3 years. There was no evidence of a difference in the OS benefit by Gleason sum score, performance status or nodal status, but the size of the benefit may vary by age. There were more grade III-IV acute cardiac, vascular and hepatic toxicities with AAP plus ADT but no excess of other toxicities or death.
Interpretation: Adding AAP to ADT is a clinically effective treatment option for men with mHSPC, offering an alternative to docetaxel for men who are starting treatment for the first time. Future research will need to address which of these two agents or whether their combination is most effective, and for whom.
Keywords: Abiraterone; Androgen deprivation therapy; Meta-analysis; Metastases; Prostate cancer; Systematic review.
Copyright © 2017 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures


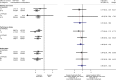
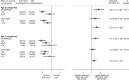
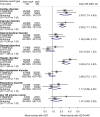
Comment in
-
Response to letter commenting on published paper: Adding abiraterone to androgen deprivation therapy in men with metastatic hormone-sensitive prostate cancer: A systematic review and meta-analysis.Eur J Cancer. 2018 May;94:218-219. doi: 10.1016/j.ejca.2018.02.002. Epub 2018 Mar 13. Eur J Cancer. 2018. PMID: 29548532 No abstract available.
-
RE: Rydzewska et al. Adding abiraterone to androgen deprivation therapy in men with metastatic hormone-sensitive prostate cancer: A systematic review and meta-analysis. Eur J Cancer. 2017 Oct; 84:88-101.Eur J Cancer. 2018 May;94:216-217. doi: 10.1016/j.ejca.2018.01.115. Epub 2018 Mar 21. Eur J Cancer. 2018. PMID: 29573942 No abstract available.
References
-
- Labrie F., Dupont A., Belanger A., Giguere M., Lacoursiere Y., Emond J. Combination therapy with flutamide and castration (LHRH agonist or orchiectomy) in advanced prostate cancer: a marked improvement in response and survival. J Steroid Biochem. 1985;23(5B):833–841. - PubMed
-
- Shiota M., Eto M. Current status of primary pharmacotherapy and future perspectives toward upfront therapy for metastatic hormone-sensitive prostate cancer. Int J Urol. 2016;23(5):360–369. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

