Expression of the RNA-binding protein RBP10 promotes the bloodstream-form differentiation state in Trypanosoma brucei
- PMID: 28800584
- PMCID: PMC5568443
- DOI: 10.1371/journal.ppat.1006560
Expression of the RNA-binding protein RBP10 promotes the bloodstream-form differentiation state in Trypanosoma brucei
Abstract
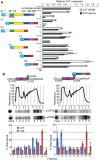
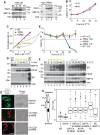
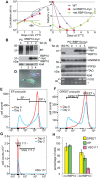
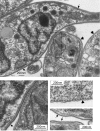
In nearly all eukaryotes, cellular differentiation is governed by changes in transcription, and stabilized by chromatin and DNA modification. Gene expression control in the pathogen Trypanosoma brucei, in contrast, relies almost exclusively on post-transcriptional mechanisms, so RNA binding proteins must assume the burden that is usually borne by transcription factors. T. brucei multiply in the blood of mammals as bloodstream forms, and in the midgut of Tsetse flies as procyclic forms. We show here that a single RNA-binding protein, RBP10, promotes the bloodstream-form trypanosome differentiation state. Depletion of RBP10 from bloodstream-form trypanosomes gives cells that can grow only as procyclic forms; conversely, expression of RBP10 in procyclic forms converts them to bloodstream forms. RBP10 binds to procyclic-specific mRNAs containing an UAUUUUUU motif, targeting them for translation repression and destruction. Products of RBP10 target mRNAs include not only the major procyclic surface protein and enzymes of energy metabolism, but also protein kinases and stage-specific RNA-binding proteins: this suggests that alterations in RBP10 trigger a regulatory cascade.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Simarro P, Cecchi G, Franco J, Paone M, Diarra A, Ruiz-Postigo J, et al. Estimating and Mapping the Population at Risk of Sleeping Sickness. PLoS Negl Trop Dis. 2012;6:e1859 doi: 10.1371/journal.pntd.0001859 - DOI - PMC - PubMed
-
- Shaw AP, Cecchi G, Wint GR, Mattioli RC, Robinson TP. Mapping the economic benefits to livestock keepers from intervening against bovine trypanosomosis in Eastern Africa. Prev Vet Med. 2014;113(2):197–210. Epub 2013/11/28. doi: 10.1016/j.prevetmed.2013.10.024 . - DOI - PubMed
-
- Dobson RJ, Dargantes AP, Mercado RT, Reid SA. Models for Trypanosoma evansi (surra), its control and economic impact on small-hold livestock owners in the Philippines. Int J Parasitol. 2009;39(10):1115–23. Epub 2009/03/11. doi: 10.1016/j.ijpara.2009.02.013 . - DOI - PubMed
-
- Fikru R, Andualem Y, Getachew T, Menten J, Hasker E, Merga B, et al. Trypanosome infection in dromedary camels in Eastern Ethiopia: Prevalence, relative performance of diagnostic tools and host related risk factors. Vet Parasitol. 2015;211(3–4):175–81. Epub 2015/06/15. doi: 10.1016/j.vetpar.2015.04.008 . - DOI - PubMed
-
- Horn D. Antigenic variation in African trypanosomes. Mol Biochem Parasitol 2014;195:123–9. doi: 10.1016/j.molbiopara.2014.05.001 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

