Cell adhesion heterogeneity reinforces tumour cell dissemination: novel insights from a mathematical model
- PMID: 28800767
- PMCID: PMC5553611
- DOI: 10.1186/s13062-017-0188-z
Cell adhesion heterogeneity reinforces tumour cell dissemination: novel insights from a mathematical model
Abstract
Background: Cancer cell invasion, dissemination, and metastasis have been linked to an epithelial-mesenchymal transition (EMT) of individual tumour cells. During EMT, adhesion molecules like E-cadherin are downregulated and the decrease of cell-cell adhesion allows tumour cells to dissociate from the primary tumour mass. This complex process depends on intracellular cues that are subject to genetic and epigenetic variability, as well as extrinsic cues from the local environment resulting in a spatial heterogeneity in the adhesive phenotype of individual tumour cells. Here, we use a novel mathematical model to study how adhesion heterogeneity, influenced by intrinsic and extrinsic factors, affects the dissemination of tumour cells from an epithelial cell population. The model is a multiscale cellular automaton that couples intracellular adhesion receptor regulation with cell-cell adhesion.
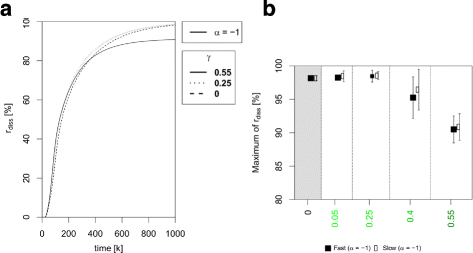
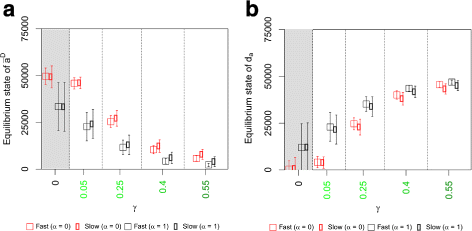
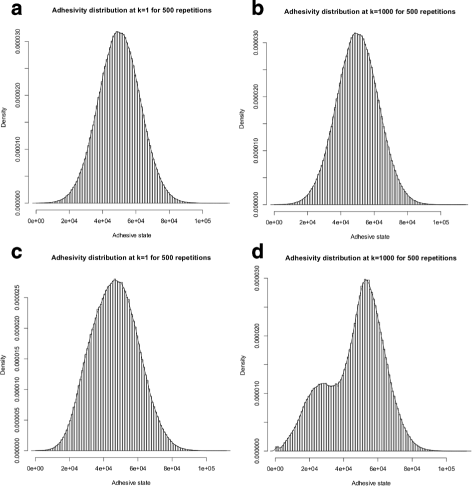
Results: Simulations of our mathematical model indicate profound effects of adhesion heterogeneity on tumour cell dissemination. In particular, we show that a large variation of intracellular adhesion receptor concentrations in a cell population reinforces cell dissemination, regardless of extrinsic cues mediated through the local cell density. However, additional control of adhesion receptor concentration through the local cell density, which can be assumed in healthy cells, weakens the effect. Furthermore, we provide evidence that adhesion heterogeneity can explain the remarkable differences in adhesion receptor concentrations of epithelial and mesenchymal phenotypes observed during EMT and might drive early dissemination of tumour cells.
Conclusions: Our results suggest that adhesion heterogeneity may be a universal trigger to reinforce cell dissemination in epithelial cell populations. This effect can be at least partially compensated by a control of adhesion receptor regulation through neighbouring cells. Accordingly, our findings explain how both an increase in intra-tumour adhesion heterogeneity and the loss of control through the local environment can promote tumour cell dissemination.
Reviewers: This article was reviewed by Hanspeter Herzel, Thomas Dandekar and Marek Kimmel.
Keywords: Cellular automaton; EMT; Intercellular adhesion; Mathematical model; Metastasis; Tumour heterogeneity; Tumour invasion.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
The role of epithelial-mesenchymal transition in cancer pathology.Pathology. 2007 Jun;39(3):305-18. doi: 10.1080/00313020701329914. Pathology. 2007. PMID: 17558857 Review.
-
From genotypes to phenotypes: classification of the tumour profiles for different variants of the cadherin adhesion pathway.Phys Biol. 2012 Jun;9(3):036008. doi: 10.1088/1478-3975/9/3/036008. Epub 2012 Jun 8. Phys Biol. 2012. PMID: 22683697
-
Modeling the influence of the E-cadherin-beta-catenin pathway in cancer cell invasion: a multiscale approach.Biophys J. 2008 Jul;95(1):155-65. doi: 10.1529/biophysj.107.114678. Epub 2008 Mar 13. Biophys J. 2008. PMID: 18339758 Free PMC article.
-
Pattern formation in a nonlocal mathematical model for the multiple roles of the TGF-β pathway in tumour dynamics.Math Biosci. 2017 Jul;289:96-115. doi: 10.1016/j.mbs.2017.05.003. Epub 2017 May 13. Math Biosci. 2017. PMID: 28511959
-
Epithelial-mesenchymal transition in cancer metastasis: mechanisms, markers and strategies to overcome drug resistance in the clinic.Biochim Biophys Acta. 2009 Dec;1796(2):75-90. doi: 10.1016/j.bbcan.2009.03.002. Epub 2009 Mar 21. Biochim Biophys Acta. 2009. PMID: 19306912 Review.
Cited by
-
Modeling How Heterogeneity in Cell Cycle Length Affects Cancer Cell Growth Dynamics in Response to Treatment.Front Oncol. 2020 Sep 10;10:1552. doi: 10.3389/fonc.2020.01552. eCollection 2020. Front Oncol. 2020. PMID: 33042800 Free PMC article.
-
Alternative splicing of ALCAM enables tunable regulation of cell-cell adhesion through differential proteolysis.Sci Rep. 2018 Feb 16;8(1):3208. doi: 10.1038/s41598-018-21467-x. Sci Rep. 2018. PMID: 29453336 Free PMC article.
-
Biophysical phenotype mixtures reveal advantages for tumor muscle invasion in vivo.Biophys J. 2023 Nov 7;122(21):4194-4206. doi: 10.1016/j.bpj.2023.09.016. Epub 2023 Sep 26. Biophys J. 2023. PMID: 37766428 Free PMC article.
-
BIO-LGCA: A cellular automaton modelling class for analysing collective cell migration.PLoS Comput Biol. 2021 Jun 15;17(6):e1009066. doi: 10.1371/journal.pcbi.1009066. eCollection 2021 Jun. PLoS Comput Biol. 2021. PMID: 34129639 Free PMC article.
-
Comprehensive Analyses of Glucose Metabolism in Glioma Reveal the Glioma-Promoting Effect of GALM.Front Cell Dev Biol. 2022 Jan 20;9:717182. doi: 10.3389/fcell.2021.717182. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35127693 Free PMC article.
References
-
- Tulotta C, He S, van der Ent W, Chen L, Groenewoud A, Spaink HP, Snaar-Jagalska BE. Imaging cancer angiogenesis and metastasis in a zebrafish embryo model. In: Langenau DM, editor. Cancer and Zebrafish: Mechanisms, Techniques, and Models. Cham: Springer; 2016. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

