Interim results from the CATNON trial (EORTC study 26053-22054) of treatment with concurrent and adjuvant temozolomide for 1p/19q non-co-deleted anaplastic glioma: a phase 3, randomised, open-label intergroup study
- PMID: 28801186
- PMCID: PMC5806535
- DOI: 10.1016/S0140-6736(17)31442-3
Interim results from the CATNON trial (EORTC study 26053-22054) of treatment with concurrent and adjuvant temozolomide for 1p/19q non-co-deleted anaplastic glioma: a phase 3, randomised, open-label intergroup study
Erratum in
-
Department of Error.Lancet. 2017 Oct 7;390(10103):1644. doi: 10.1016/S0140-6736(17)32438-8. Epub 2017 Oct 5. Lancet. 2017. PMID: 29131794 No abstract available.
Abstract
Background: The role of temozolomide chemotherapy in newly diagnosed 1p/19q non-co-deleted anaplastic gliomas, which are associated with lower sensitivity to chemotherapy and worse prognosis than 1p/19q co-deleted tumours, is unclear. We assessed the use of radiotherapy with concurrent and adjuvant temozolomide in adults with non-co-deleted anaplastic gliomas.
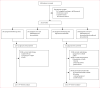
Methods: This was a phase 3, randomised, open-label study with a 2 × 2 factorial design. Eligible patients were aged 18 years or older and had newly diagnosed non-co-deleted anaplastic glioma with WHO performance status scores of 0-2. The randomisation schedule was generated with the electronic EORTC web-based ORTA system. Patients were assigned in equal numbers (1:1:1:1), using the minimisation technique, to receive radiotherapy (59·4 Gy in 33 fractions of 1·8 Gy) alone or with adjuvant temozolomide (12 4-week cycles of 150-200 mg/m2 temozolomide given on days 1-5); or to receive radiotherapy with concurrent temozolomide 75 mg/m2 per day, with or without adjuvant temozolomide. The primary endpoint was overall survival adjusted for performance status score, age, 1p loss of heterozygosity, presence of oligodendroglial elements, and MGMT promoter methylation status, analysed by intention to treat. We did a planned interim analysis after 219 (41%) deaths had occurred to test the null hypothesis of no efficacy (threshold for rejection p<0·0084). This trial is registered with ClinicalTrials.gov, number NCT00626990.
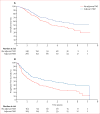
Findings: At the time of the interim analysis, 745 (99%) of the planned 748 patients had been enrolled. The hazard ratio for overall survival with use of adjuvant temozolomide was 0·65 (99·145% CI 0·45-0·93). Overall survival at 5 years was 55·9% (95% CI 47·2-63·8) with and 44·1% (36·3-51·6) without adjuvant temozolomide. Grade 3-4 adverse events were seen in 8-12% of 549 patients assigned temozolomide, and were mainly haematological and reversible.
Interpretation: Adjuvant temozolomide chemotherapy was associated with a significant survival benefit in patients with newly diagnosed non-co-deleted anaplastic glioma. Further analysis of the role of concurrent temozolomide treatment and molecular factors is needed.
Funding: Schering Plough and MSD.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The other authors declare no competing interests.
Figures
Comment in
-
Benefit with adjuvant chemotherapy in anaplastic astrocytoma.Lancet. 2017 Oct 7;390(10103):1625-1626. doi: 10.1016/S0140-6736(17)31477-0. Epub 2017 Aug 8. Lancet. 2017. PMID: 28801187 No abstract available.
-
CATNON interim results: another triumph of upfront chemotherapy in glioma.Neuro Oncol. 2017 Oct 1;19(10):1287-1288. doi: 10.1093/neuonc/nox124. Neuro Oncol. 2017. PMID: 28922864 Free PMC article. No abstract available.
-
Treatment of WHO Grade 2 and 3 Gliomas With Potentially Favorable Survival: Is Monotherapy Obsolete?Int J Radiat Oncol Biol Phys. 2019 Mar 1;103(3):533-536. doi: 10.1016/j.ijrobp.2018.08.025. Epub 2019 Feb 1. Int J Radiat Oncol Biol Phys. 2019. PMID: 31088778 No abstract available.
References
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. - PubMed
-
- Hegi ME, Diserens A-C, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. - PubMed
-
- van den Bent MJ, Carpentier AF, Brandes AA, et al. Adjuvant PCV improves progression free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: a randomized EORTC phase III trial. J Clin Oncol. 2006;24:2715–22. - PubMed
-
- Cairncross JG, Berkey B, Shaw E, et al. Phase III trial of chemotherapy plus radiotherapy (RT) versus RT alone for pure and mixed anaplastic oligodendroglioma (RTOG 9402): an intergroup trial by the RTOG, NCCTG, SWOG, NCI CTG and ECOG. J Clin Oncol. 2006;24:2707–14. - PubMed
-
- Cairncross JG, Ueki K, Zlatescu MC, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90:1473–79. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

