Disrupting glutamine metabolic pathways to sensitize gemcitabine-resistant pancreatic cancer
- PMID: 28801576
- PMCID: PMC5554139
- DOI: 10.1038/s41598-017-08436-6
Disrupting glutamine metabolic pathways to sensitize gemcitabine-resistant pancreatic cancer
Abstract
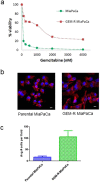
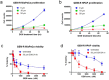
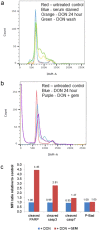
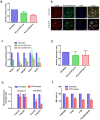
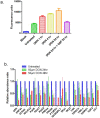
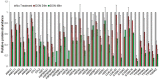
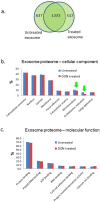
Pancreatic cancer is a lethal disease with poor prognosis. Gemcitabine has been the first line systemic treatment for pancreatic cancer. However, the rapid development of drug resistance has been a major hurdle in gemcitabine therapy leading to unsatisfactory patient outcomes. With the recent renewed understanding of glutamine metabolism involvement in drug resistance and immuno-response, we investigated the anti-tumor effect of a glutamine analog (6-diazo-5-oxo-L-norleucine) as an adjuvant treatment to sensitize chemoresistant pancreatic cancer cells. We demonstrate that disruption of glutamine metabolic pathways improves the efficacy of gemcitabine treatment. Such a disruption induces a cascade of events which impacts glycan biosynthesis through Hexosamine Biosynthesis Pathway (HBP), as well as cellular redox homeostasis, resulting in global changes in protein glycosylation, expression and functional effects. The proteome alterations induced in the resistant cancer cells and the secreted exosomes are intricately associated with the reduction in cell proliferation and the enhancement of cancer cell chemosensitivity. Proteins associated with EGFR signaling, including downstream AKT-mTOR pathways, MAPK pathway, as well as redox enzymes were downregulated in response to disruption of glutamine metabolic pathways.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

