Deficiency in catechol-o-methyltransferase is linked to a disruption of glucose homeostasis in mice
- PMID: 28801594
- PMCID: PMC5554180
- DOI: 10.1038/s41598-017-08513-w
Deficiency in catechol-o-methyltransferase is linked to a disruption of glucose homeostasis in mice
Abstract
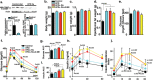
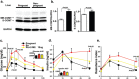
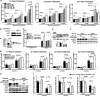
2-methoxyestradiol (2-ME), an estrogen metabolite generated via catechol-o-methyltransferase (COMT), is multifunctional methoxy-catechol. Here, we report that COMT deficiency leads to glucose intolerance and 2-ME rescues COMT-deficient-associated metabolic defects. Liver COMT protein was suppressed in high fat diet (HFD)-fed or in pregnant mice. COMT suppression, by Ro41-0960 or siRNA, in HFD fed mice or in pregnant mice exacerbated glucose intolerance; 2-ME intervention ameliorated these defects. 2-ME effects on glucose tolerance were associated with AMPK phosphorylation in the liver and in islet cells. Metformin restored liver COMT protein levels, and metformin-induced liver AMPK phosphorylation was abolished by COMT inhibition. The amelioration in glucose tolerance by 2-ME was associated with biphasic insulin secretion in an environment-dependent manner. 2-ME-induced insulin secretion was associated with the AMPK phosphorylation, PDX-1 phosphorylation, and MST-1 suppression in MIN-6 cells. Furthermore 2-ME displayed PPARγ agonist-like activity. These results suggest that COMT is an enzyme to maintain glucose homeostasis and 2-ME is a potential endogenous multi-target anti-diabetic candidate.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Tunbridge, E. M., Harrison, P. J. & Weinberger, D. R. Catechol-o-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol Psychiatry. 60, 141–151. Epub 2006 Feb 2014 (2006). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

