Selective effects of non-thermal atmospheric plasma on triple-negative breast normal and carcinoma cells through different cell signaling pathways
- PMID: 28801613
- PMCID: PMC5554176
- DOI: 10.1038/s41598-017-08792-3
Selective effects of non-thermal atmospheric plasma on triple-negative breast normal and carcinoma cells through different cell signaling pathways
Abstract
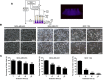
Non-thermal atmospheric plasma (NTP) has shown its selective anticancer effects in many types of tumors in vitro and one of the main mechanisms is that the different increase of intracellular ROS in cancer and homologous normal cells. In this study, we report that NTP treatment reduces the proliferation in triple negative breast cancer (TNBC) and normal cell lines. Simultaneously, STAT3 pathway is inhibited by NTP effects. However, it is observed that normal cells MCF10A are more sensitive to ROS toxicity induced by NTP than cancer cells MDA-MB-231. When 5 mM of ROS inhibitor N-acetyl cysteine (NAC) is employed in NTP treatments, the proliferation of normal breast cells MCF10A recovers. Meanwhile, NTP effects remain significant inhibition of MDA-MB-231 cells. Our results further reveal that NTP can induce apoptosis in MDA-MB-231 cells through inhibiting interleukin-6 receptor (IL-6R) pathway. Moreover, the mechanism of NTP anti-cancer selectivity relates to constantly HER2/Akt activation induced by NTP especially in MCF10A cells but not in MDA-MB-231 cells. Therefore, these two different cell signaling pathways induced by NTP treatments in TNBC and homologous normal cells make NTP becoming a potential tool in future therapy.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

