Evaluation of novel factor Xa inhibitors from Oxya chinensis sinuosa with anti-platelet aggregation activity
- PMID: 28801633
- PMCID: PMC5554137
- DOI: 10.1038/s41598-017-08330-1
Evaluation of novel factor Xa inhibitors from Oxya chinensis sinuosa with anti-platelet aggregation activity
Abstract
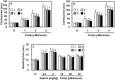
The edible grasshopper Oxya chinensis sinuosa is consumed worldwide for its various medicinal effects. The purpose of this study was to investigate potential bioactive antithrombotic and antiplatelet compounds from O. chinensis sinuosa. Five N-acetyldopamine dimers (1-5) were isolated from O. chinensis sinuosa and compounds 1 and 2 were identified as new chemicals with chiral centers at H-2 and H-3 of the benzo-1,4-dioxane structure. Compounds 1-4 were found to have both FXa and platelet aggregation inhibitory activities. These compounds inhibited the catalytic activity of FXa toward its synthetic substrate, S-2222, by noncompetitive inhibition, and inhibited platelet aggregation induced by ADP and U46619. Furthermore, compounds 1-4 showed enhanced antithrombotic effects, which were assessed using in vivo models of pulmonary embolism and arterial thrombosis. The isolated compounds also showed anticoagulant effects in mice. However, compounds 1-4 did not prolong bleeding time in mice, as shown by tail clipping. N-Acetyldopamine dimers, including two new stereoisomers 1 and 2, are novel antithrombotic compounds showing both FXa inhibition and antiplatelet aggregation activity with a low bleeding risk. Collectively, these results suggest that compounds 1-4 could serve as candidates and provide scaffolds for development of new antithrombotic drugs.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


 ) correlations of compounds 1 and 2.
) correlations of compounds 1 and 2.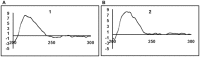



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

