BiVO4-rGO with a novel structure on steel fabric used as high-performance photocatalysts
- PMID: 28801660
- PMCID: PMC5554144
- DOI: 10.1038/s41598-017-07342-1
BiVO4-rGO with a novel structure on steel fabric used as high-performance photocatalysts
Erratum in
-
Author Correction: BiVO4-rGO with a novel structure on steel fabric used as high-performance photocatalysts.Sci Rep. 2020 Mar 24;10(1):5669. doi: 10.1038/s41598-020-62437-6. Sci Rep. 2020. PMID: 32205846 Free PMC article.
Abstract
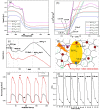
A high-performance and novel photocatalyst of BiVO4-reduced Graphene Oxide (BiVO4-rGO) nanocomposite was prepared by a facile hydrothermal method. The photocatalyst was characterized by X-ray diffraction, X-ray photoelectron spectroscopy, scanning electron microscopy, transmission electronic microscopy, UV-Vis diffusion reflectance spectroscopy, photoluminescence spectroscopy and UV-Vis adsorption spectroscopy, respectively. The visible-light photocatalytic activity was evaluated by oxidation of methyl orange (MO) under simulated sunlight irradiation. The results show that the BiVO4-rGO nanocomposites exhibit enhanced photocatalytic performance for the degradation of MO with a maximum removal rate of 98.95% under visible light irradiation as compared with pure BiVO4 (57.55%) due to the increased light absorption intensity and the degradation of electron-hole pair recombination in BiVO4 with the introduction of the rGO.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Hisaindee S, et al. Application of LC-MS to the analysis of advanced oxidation process (AOP) degradation of dye products and reaction mechanisms. Trends. Anal. Chem. 2013;49:31–44. doi: 10.1016/j.trac.2013.03.011. - DOI
-
- Rauf MA, et al. Adsorption of dyes from aqueous solutions onto sand and their kinetic behavior. Chem. Eng. J. 2008;137:238–243. doi: 10.1016/j.cej.2007.04.025. - DOI
-
- Jamal F, et al. Biocatalytic activity of immobilized pointed gourd (Trichosanthes dioica) peroxidase-concanavalin A complex on calcium alginate pectin gel. J. Mol. Catal. B. Enzym. 2012;74:125–131. doi: 10.1016/j.molcatb.2011.09.008. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

