Antibiofilm Effect of Poly(Vinyl Alcohol- co-Ethylene) Halamine Film against Listeria innocua and Escherichia coli O157:H7
- PMID: 28802271
- PMCID: PMC5601348
- DOI: 10.1128/AEM.00975-17
Antibiofilm Effect of Poly(Vinyl Alcohol- co-Ethylene) Halamine Film against Listeria innocua and Escherichia coli O157:H7
Abstract
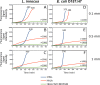
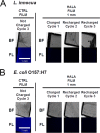
Bacterial biofilm formation is linked to several infections and foodborne disease outbreaks. To address this challenge, there is an unmet need to develop rechargeable antimicrobial materials that can provide continuous sanitation of contact surfaces, especially in the food industry. This study was aimed at evaluating a novel rechargeable antimicrobial polymer formed using poly(vinyl alcohol-co-ethylene) (PVA-co-PE) with halamine functionality to prevent biofilm formation with repeated exposure to high loads of bacteria and organic content and also to aid in inactivation of preformed biofilms upon contact with this novel material. The antibiofilm activity of this rechargeable antimicrobial material was evaluated using a combination of fluorescence and scanning electron microscopy techniques and biofilm metabolic activity analyses. The results determined on the basis of imaging and metabolic activity measurements demonstrated that halamine-functionalized polymer films significantly reduced Listeria innocua and Escherichia coli O157:H7 biofilm formation. This novel polymeric material maintained its antibiofilm activity with repeated cycles of extended exposure to high levels of bacterial load. These polymeric films were recharged using bleach and cleaned using mechanical sonication after each cycle of extended incubation with bacteria. Halamine-functionalized polymeric material also exhibited significant antibacterial activity against preformed biofilms on a model surface. In summary, our results demonstrate the potential of this antimicrobial material to provide continuous sanitation of surfaces and applications for inactivating preformed biofilms without extensive use of resources, including water and heat. This polymeric material may be used as a replacement for existing polymeric materials or as a coating on diverse materials.IMPORTANCE Conventional sanitizers can have limited efficacy in inactivating biofilms in areas with limited accessibility and buildup of organic biomass. Furthermore, none of the current approaches provide continuous sanitation of surfaces. There is a significant unmet need to develop and validate materials that can prevent biofilm formation as well as inactivate preformed biofilms. In this study, the efficacy of a copolymer film containing N-halamine against biofilms of L. innocua and E. coli O157:H7 was evaluated. The polymer film showed strong inhibitory activity against pregrown biofilm or prevented the growth of a new biofilm. The polymer film also maintained its antibiofilm activity after multiple cycles of exposure to high titers of bacterial load with recharging of the polymer film using bleach at intermediate steps between the cycles. Overall, the results demonstrate the potential of a novel antimicrobial material to inhibit and treat biofilms in food industry applications.
Keywords: Escherichia coli O157:H7; Listeria innocua; antimicrobial polymers; biofilm; halamines; sanitation.
Copyright © 2017 American Society for Microbiology.
Figures





References
-
- de Souza EL, Meira QGS, de Medeiros Barbosa I, Athayde AJAA, da Conceição ML, de Siqueira Júnior JP. 2014. Biofilm formation by Staphylococcus aureus from food contact surfaces in a meat-based broth and sensitivity to sanitizers. Braz J Microbiol 45:67–75. doi: 10.1590/S1517-83822014000100010. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

