Evidence for Borrelia bavariensis Infections of Ixodes uriae within Seabird Colonies of the North Atlantic Ocean
- PMID: 28802273
- PMCID: PMC5626983
- DOI: 10.1128/AEM.01087-17
Evidence for Borrelia bavariensis Infections of Ixodes uriae within Seabird Colonies of the North Atlantic Ocean
Abstract
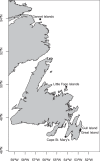
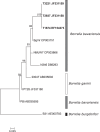
The first report of members of the spirochete genus Borrelia in the seabird tick, Ixodes uriae, and seabird colonies occurred during the early 1990s. Since then, Borrelia spp. have been detected in these ticks and seabird colonies around the world. To date, the primary species detected has been Borrelia garinii, with rare occurrences of Borrelia burgdorferi sensu stricto and Borrelia lusitaniae. During our research on Borrelia and I. uriae in seabird colonies of Newfoundland and Labrador, Canada, we have identified Borrelia bavariensis in I. uriae To our knowledge, B. bavariensis has previously been found only in the Eurasian tick species Ixodes persulcatus and Ixodes ricinus, and it was believed to be a rodent-specific Borrelia ecotype. We found B. bavariensis within I. uriae from three seabird colonies over three calendar years. We also reanalyzed B. garinii sequences collected from I. uriae from Eurasian seabird colonies and determined that sequences from two Russian seabird colonies likely also represent B. bavariensis The Canadian B. bavariensis sequences from I. uriae analyzed in this study cluster with previously described sequences from Asia. Overall, this is an important discovery that illustrates and expands the range of hosts and vectors for B. bavariensis, and it raises questions regarding the possible mechanisms of pathogen dispersal from Asia to North America.IMPORTANCE To our knowledge, this is the first documentation of B. bavariensis outside Eurasia. Additionally, the bacterium was found in a marine ecosystem involving the seabird tick I. uriae and its associated seabird hosts. This indicates that the epizootiology of B. bavariensis transmission is much different from what had been described, with this species previously believed to be a rodent-specific ecotype, and it indicates that this pathogen is established, or establishing, much more widely.
Keywords: Borrelia; Lyme disease; MLST; seabird; tick-borne pathogens.
© Crown copyright 2017.
Figures

References
-
- Piesman J, Gern L. 2004. Lyme borreliosis in Europe and North America. Parasitology 129(Suppl):S191–S220. - PubMed
-
- Fukunaga M, Takahashi Y, Tsuruta Y, Matsushita O, Ralph D, McClelland M, Nakao M. 1995. Genetic and phenotypic analysis of Borrelia miyamotoi sp. nov., isolated from the ixodid tick Ixodes persulcatus, the vector for Lyme disease in Japan. Int J Syst Bacteriol 45:804–810. doi: 10.1099/00207713-45-4-804. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

