Cardiac neuroanatomy - Imaging nerves to define functional control
- PMID: 28802636
- PMCID: PMC5680093
- DOI: 10.1016/j.autneu.2017.07.008
Cardiac neuroanatomy - Imaging nerves to define functional control
Abstract
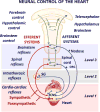
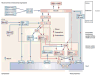
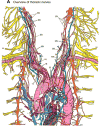
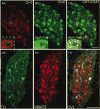
The autonomic nervous system regulates normal cardiovascular function and plays a critical role in the pathophysiology of cardiovascular disease. Further understanding of the interplay between the autonomic nervous system and cardiovascular system holds promise for the development of neuroscience-based cardiovascular therapeutics. To this end, techniques to image myocardial innervation will help provide a basis for understanding the fundamental underpinnings of cardiac neural control. In this review, we detail the evolution of gross and microscopic anatomical studies for functional mapping of cardiac neuroanatomy.
Keywords: Autonomic nervous system; Intracardiac nervous system; Myocardial innervation; Neurocardiology.
Copyright © 2017 Elsevier B.V. All rights reserved.
Figures


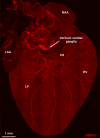

References
-
- Jänig W. Integrative Action of the Autonomic Nervous System: Neurobiology of Homeostasis. Cambridge University Press; 2008.
-
- Barron HV, Lesh MD. Autonomic nervous system and sudden cardiac death. J. Am. Coll. Cardiol. 1996;27:1053–1060. - PubMed
-
- Nolan J, et al. Prospective study of heart rate variability and mortality in chronic heart failure: results of the United Kingdom heart failure evaluation and assessment of risk trial (UK-heart) Circulation. 1998;98:1510–1516. - PubMed
-
- La Rovere MT, Bigger JT, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet Lond. Engl. 1998;351:478–484. - PubMed
-
- Floras JS. Sympathetic activation in human heart failure: diverse mechanisms, therapeutic opportunities. Acta Physiol. Scand. 2003;177:391–398. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

