Nanoparticle-releasing nanofiber composites for enhanced in vivo vaginal retention
- PMID: 28802690
- PMCID: PMC5599218
- DOI: 10.1016/j.biomaterials.2017.07.034
Nanoparticle-releasing nanofiber composites for enhanced in vivo vaginal retention
Abstract
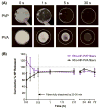
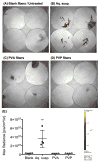
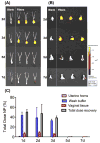
Current approaches for topical vaginal administration of nanoparticles result in poor retention and extensive leakage. To overcome these challenges, we developed a nanoparticle-releasing nanofiber delivery platform and evaluated its ability to improve nanoparticle retention in a murine model. We individually tailored two components of this drug delivery system for optimal interaction with mucus, designing (1) mucoadhesive fibers for better retention in the vaginal tract, and (2) PEGylated nanoparticles that diffuse quickly through mucus. We hypothesized that this novel dual-functioning (mucoadhesive/mucus-penetrating) composite material would provide enhanced retention of nanoparticles in the vaginal mucosa. Equivalent doses of fluorescent nanoparticles were vaginally administered to mice in either water (aqueous suspension) or fiber composites, and fluorescent content was quantified in cervicovaginal mucus and vaginal tissue at time points from 24 h to 7d. We also fabricated composite fibers containing etravirine-loaded nanoparticles and evaluated the pharmacokinetics over 7d. We found that our composite materials provided approximately 30-fold greater retention of nanoparticles in the reproductive tract at 24 h compared to aqueous suspensions. Compared to nanoparticles in aqueous suspension, the nanoparticles in fiber composites exhibited sustained and higher etravirine concentrations after 24 h and up to 7d, demonstrating the capabilities of this new delivery platform to sustain nanoparticle release out to 3d and drug retention out to one week after a single administration. This is the first report of nanoparticle-releasing fibers for vaginal drug delivery, as well as the first study of a single delivery system that combines two components uniquely engineered for complementary interactions with mucus.
Keywords: Electrospinning; Microbicides; Nanofibers; Nanoparticles; Pharmacokinetics; Vaginal drug delivery.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures




Similar articles
-
Vaginal drug delivery: strategies and concerns in polymeric nanoparticle development.Expert Opin Drug Deliv. 2014 Sep;11(9):1419-34. doi: 10.1517/17425247.2014.924499. Epub 2014 Jun 24. Expert Opin Drug Deliv. 2014. PMID: 24960192 Review.
-
Modeling of nanoparticle transport through the female reproductive tract for the treatment of infectious diseases.Eur J Pharm Biopharm. 2019 May;138:37-47. doi: 10.1016/j.ejpb.2018.09.003. Epub 2018 Sep 7. Eur J Pharm Biopharm. 2019. PMID: 30195726
-
Mucus-penetrating nanoparticles for vaginal drug delivery protect against herpes simplex virus.Sci Transl Med. 2012 Jun 13;4(138):138ra79. doi: 10.1126/scitranslmed.3003453. Sci Transl Med. 2012. PMID: 22700955 Free PMC article.
-
Development and characterization of chitosan nanoparticles loaded nanofiber hybrid system for vaginal controlled release of benzydamine.Eur J Pharm Sci. 2021 Jun 1;161:105801. doi: 10.1016/j.ejps.2021.105801. Epub 2021 Mar 7. Eur J Pharm Sci. 2021. PMID: 33691155
-
Vaginal mucoadhesive drug delivery systems.Drug Dev Ind Pharm. 2012 Jun;38(6):643-52. doi: 10.3109/03639045.2011.623355. Epub 2011 Oct 15. Drug Dev Ind Pharm. 2012. PMID: 21999572 Review.
Cited by
-
Electrospun nanofibers: Focus on local therapeutic delivery targeting infectious disease.J Drug Deliv Sci Technol. 2025 Feb;104:106520. doi: 10.1016/j.jddst.2024.106520. Epub 2024 Dec 20. J Drug Deliv Sci Technol. 2025. PMID: 39802685
-
Spun Biotextiles in Tissue Engineering and Biomolecules Delivery Systems.Antibiotics (Basel). 2020 Apr 12;9(4):174. doi: 10.3390/antibiotics9040174. Antibiotics (Basel). 2020. PMID: 32290536 Free PMC article. Review.
-
Mesh and layered electrospun fiber architectures as vehicles for Lactobacillus acidophilus and Lactobacillus crispatus intended for vaginal delivery.Biomater Adv. 2023 Nov;154:213614. doi: 10.1016/j.bioadv.2023.213614. Epub 2023 Aug 29. Biomater Adv. 2023. PMID: 37659215 Free PMC article.
-
Pharmaceutical Vehicles for Vaginal and Rectal Administration of Anti-HIV Microbicide Nanosystems.Pharmaceutics. 2019 Mar 26;11(3):145. doi: 10.3390/pharmaceutics11030145. Pharmaceutics. 2019. PMID: 30917532 Free PMC article. Review.
-
Reverse Transcriptase Inhibitors Nanosystems Designed for Drug Stability and Controlled Delivery.Pharmaceutics. 2019 Apr 27;11(5):197. doi: 10.3390/pharmaceutics11050197. Pharmaceutics. 2019. PMID: 31035595 Free PMC article. Review.
References
-
- Yang M, Yu T, Wang YY, Lai SK, Zeng Q, Miao B, Tang BC, Simons BW, Ensign LM, Liu G, Chan KWY, Juang CY, Mert O, Wood J, Fu J, McMahon MT, Wu TC, Hung CF, Hanes J. Vaginal Delivery of Paclitaxel via Nanoparticles with Non-Mucoadhesive Surfaces Suppresses Cervical Tumor Growth. Adv Healthc Mater. 2014;3:1044–1052. doi: 10.1002/adhm.201300519. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

