Thoracic Oncology Clinical Trial Eligibility Criteria and Requirements Continue to Increase in Number and Complexity
- PMID: 28802905
- PMCID: PMC5610621
- DOI: 10.1016/j.jtho.2017.07.020
Thoracic Oncology Clinical Trial Eligibility Criteria and Requirements Continue to Increase in Number and Complexity
Abstract
Introduction: Eligibility criteria and screening procedures are designed to optimize the scientific yield and maximize the safety of clinical trials. However, they may also heighten trial complexity, hinder enrollment, decrease generalizability, and increase costs. We analyzed the types and number of eligibility criteria and screening procedures among thoracic oncology clinical trials sponsored or endorsed by the Eastern Cooperative Oncology Group.
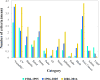
Methods: We identified trials and obtained protocols from the Eastern Cooperative Oncology Group website. Eligibility criteria were grouped and categorized as comorbidity (classified by organ system), administrative requirements, prior treatment, and measurable disease requirements. Associations between trial characteristics and eligibility criteria were analyzed by using the Kruskal-Wallis and Wilcoxon tests.
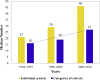
Results: A total of 74 lung cancer trials activated in 1986-2016 were identified. The total number of eligibility criteria was associated with trial principal therapy (a median of nine for surgical, 18 for radiation, and 20 for medical therapy [p = 0.02]), trial primary end point (a median of 20 for overall survival, 28 for progression-free survival, and 17 for other [p = 0.001]), number of therapies (p = 0.05), and year of activation (a median of 16 for 1986-1995, 19 for 1996-2005, and 27 for 2006-2016 [P < 0.001]). The increase in trial eligibility requirements over time was limited to medical therapy trials. Over time, there was also an increase in blood test screening procedures (p = 0.05) but not in imaging, cardiac assessment, or pulmonary function screening procedures.
Conclusions: The number of eligibility criteria and screening procedures in medical therapy lung cancer clinical trials continues to rise. Continued efforts to simplify protocol eligibility and procedures are warranted to promote trial adherence, enrollment, completion, and generalizability.
Keywords: Accrual; Clinical research; Enrollment; Exclusion criteria; Inclusion criteria; Lung cancer.
Copyright © 2017 International Association for the Study of Lung Cancer. Published by Elsevier Inc. All rights reserved.
Figures
Comment in
-
The Ever-Increasing Number of Trial Eligibility Criteria: Time to Bend the Curve.J Thorac Oncol. 2017 Oct;12(10):1459-1460. doi: 10.1016/j.jtho.2017.08.003. J Thorac Oncol. 2017. PMID: 28939144 No abstract available.
-
Another Drawback for Clinical Trials Entry Criteria.J Thorac Oncol. 2018 Jul;13(7):e116-e117. doi: 10.1016/j.jtho.2018.02.007. J Thorac Oncol. 2018. PMID: 29935847 No abstract available.
References
-
- Friedman MA, Cain DF. National Cancer Institute sponsored cooperative clinical trials. Cancer. 1990;65:2376–82. - PubMed
-
- Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291:2720–6. - PubMed
-
- Tournoux C, Katsahian S, Chevret S, et al. Factors influencing inclusion of patients with malignancies in clinical trials. Cancer. 2006;106:258–70. - PubMed
-
- Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US Food and Drug Administration. J Clin Oncol. 2004;22:4626–31. - PubMed
-
- Howerton MW, Gibbons MC, Baffi CR, et al. Provider roles in the recruitment of underrepresented populations to cancer clinical trials. Cancer. 2007;109:465–76. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical