Melatonin, mitochondria, and the skin
- PMID: 28803347
- PMCID: PMC5693733
- DOI: 10.1007/s00018-017-2617-7
Melatonin, mitochondria, and the skin
Abstract
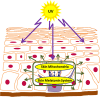
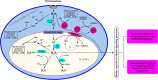
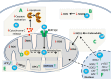
The skin being a protective barrier between external and internal (body) environments has the sensory and adaptive capacity to maintain local and global body homeostasis in response to noxious factors. An important part of the skin response to stress is its ability for melatonin synthesis and subsequent metabolism through the indolic and kynuric pathways. Indeed, melatonin and its metabolites have emerged as indispensable for physiological skin functions and for effective protection of a cutaneous homeostasis from hostile environmental factors. Moreover, they attenuate the pathological processes including carcinogenesis and other hyperproliferative/inflammatory conditions. Interestingly, mitochondria appear to be a central hub of melatonin metabolism in the skin cells. Furthermore, substantial evidence has accumulated on the protective role of the melatonin against ultraviolet radiation and the attendant mitochondrial dysfunction. Melatonin and its metabolites appear to have a modulatory impact on mitochondrion redox and bioenergetic homeostasis, as well as the anti-apoptotic effects. Of note, some metabolites exhibit even greater impact than melatonin alone. Herein, we emphasize that melatonin-mitochondria axis would control integumental functions designed to protect local and perhaps global homeostasis. Given the phylogenetic origin and primordial actions of melatonin, we propose that the melatonin-related mitochondrial functions represent an evolutionary conserved mechanism involved in cellular adaptive response to skin injury and repair.
Keywords: Homeostasis; Melatonin; Mitochondria; Photoprotection; Skin.
Figures



References
-
- Bolognia J. Dermatology. 2. Philadelphia: Mosby Elsevier; 2008.
-
- Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;21:457–487. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

