Solasodine inhibits human colorectal cancer cells through suppression of the AKT/glycogen synthase kinase-3β/β-catenin pathway
- PMID: 28803443
- PMCID: PMC5666038
- DOI: 10.1111/cas.13354
Solasodine inhibits human colorectal cancer cells through suppression of the AKT/glycogen synthase kinase-3β/β-catenin pathway
Abstract
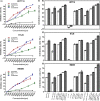
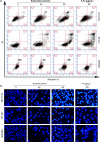
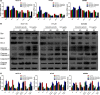
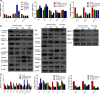
Solasodine is a main active component isolated from Solanum incanum L. that performs a wide range of functions containing anti-oxidant, anti-infection, and neurogenesis promotion. In this study, we explored the influence of solasodine on three types of human colorectal cancer (CRC) cell lines. The results show that solasodine prohibited CRC cell proliferation dose- and time-dependently and impeded CRC cell motility by downregulating MMPs. Solasodine was also found to fuel caspase-cascade reaction and increase the ratio between Bax and Bcl-2 so as to induce CRC cell apoptosis. When cells were pretreated with AKT activator (insulin-like growth factor-1) followed by solasodine, the solasodine-induced apoptosis was partially abrogated by insulin-like growth factor-1. Moreover, solasodine hindered tumor development and stimulated similar mechanisms in vivo. In general, our study provides the first evidence that solasodine has a suppressive effect on CRC cells and that this agent may be a novel therapeutic drug for CRC treatment.
Keywords: Apoptosis; colorectal cancer; metastasis; solasodine; β-Catenin.
© 2017 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures










References
-
- Ferlay J, Ervik M, Dikshit R et al Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–86. - PubMed
-
- Ece E, Suayib Y. Maintenance strategy in metastatic colorectal cancer: a systematic review. Cancer Treat Rev 2016; 42: 82–90. - PubMed
-
- Kim KY, Cha IH, Ahn JB et al Estimating the adjuvant chemotherapy effect in elderly stage II and III colon cancer patients in an observational study. J Surg Oncol 2013; 107: 613–8. - PubMed
-
- Goss KH, Groden J. Biology of the adenomatous polyposis coli tumor suppressor. J Clin Oncol 2000; 18: 1967–79. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

