Reck and Gpr124 Are Essential Receptor Cofactors for Wnt7a/Wnt7b-Specific Signaling in Mammalian CNS Angiogenesis and Blood-Brain Barrier Regulation
- PMID: 28803732
- PMCID: PMC5586543
- DOI: 10.1016/j.neuron.2017.07.031
Reck and Gpr124 Are Essential Receptor Cofactors for Wnt7a/Wnt7b-Specific Signaling in Mammalian CNS Angiogenesis and Blood-Brain Barrier Regulation
Erratum in
-
Reck and Gpr124 Are Essential Receptor Cofactors for Wnt7a/Wnt7b-Specific Signaling in Mammalian CNS Angiogenesis and Blood-Brain Barrier Regulation.Neuron. 2017 Aug 30;95(5):1221-1225. doi: 10.1016/j.neuron.2017.08.032. Neuron. 2017. PMID: 28858622 No abstract available.
Abstract
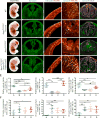
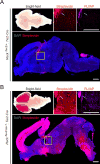
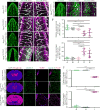
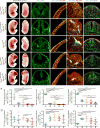
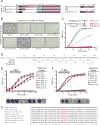
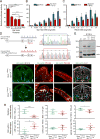
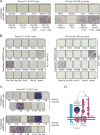
Reck, a GPI-anchored membrane protein, and Gpr124, an orphan GPCR, have been implicated in Wnt7a/Wnt7b signaling in the CNS vasculature. We show here that vascular endothelial cell (EC)-specific reduction in Reck impairs CNS angiogenesis and that EC-specific postnatal loss of Reck, combined with loss of Norrin, impairs blood-brain barrier (BBB) maintenance. The most N-terminal domain of Reck binds to the leucine-rich repeat (LRR) and immunoglobulin (Ig) domains of Gpr124, and weakening this interaction by targeted mutagenesis reduces Reck/Gpr124 stimulation of Wnt7a signaling in cell culture and impairs CNS angiogenesis. Finally, a soluble Gpr124(LRR-Ig) probe binds to cells expressing Frizzled, Wnt7a or Wnt7b, and Reck, and a soluble Reck(CC1-5) probe binds to cells expressing Frizzled, Wnt7a or Wnt7b, and Gpr124. These experiments indicate that Reck and Gpr124 are part of the cell surface protein complex that transduces Wnt7a- and Wnt7b-specific signals in mammalian CNS ECs to promote angiogenesis and regulate the BBB.
Keywords: CNS angiogenesis; Wnt reporter; blood-brain barrier; canonical Wnt signaling; mouse genetics; vascular endothelial cells.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

