Epidermal E-Cadherin Dependent β-Catenin Pathway Is Phytochemical Inducible and Accelerates Anagen Hair Cycling
- PMID: 28803863
- PMCID: PMC5675464
- DOI: 10.1016/j.ymthe.2017.07.010
Epidermal E-Cadherin Dependent β-Catenin Pathway Is Phytochemical Inducible and Accelerates Anagen Hair Cycling
Abstract
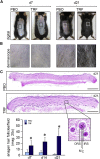
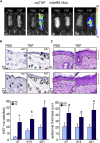
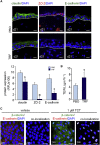
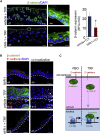
Unlike the epidermis, which regenerates continually, hair follicles anchored in the subcutis periodically regenerate by spontaneous repetitive cycles of growth (anagen), degeneration (catagen), and rest (telogen). The loss of hair follicles in response to injuries or pathologies such as alopecia endangers certain inherent functions of the skin. Thus, it is of interest to understand mechanisms underlying follicular regeneration in adults. In this work, a phytochemical rich in the natural vitamin E tocotrienol (TRF) served as a productive tool to unveil a novel epidermal pathway of hair follicular regeneration. Topical TRF application markedly induced epidermal hair follicle development akin to that during fetal skin development. This was observed in the skin of healthy as well as diabetic mice, which are known to be resistant to anagen hair cycling. TRF suppressed epidermal E-cadherin followed by 4-fold induction of β-catenin and its nuclear translocation. Nuclear β-catenin interacted with Tcf3. Such sequestration of Tcf3 from its otherwise known function to repress pluripotent factors induced the plasticity factors Oct4, Sox9, Klf4, c-Myc, and Nanog. Pharmacological inhibition of β-catenin arrested anagen hair cycling by TRF. This work reports epidermal E-cadherin/β-catenin as a novel pathway capable of inducing developmental folliculogenesis in the adult skin.
Keywords: E-cadherin; anagen; hair follicles; stem cells; β-catenin.
Copyright © 2017 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

