Impact of Prostatic-specific Antigen Threshold and Screening Interval in Prostate Cancer Screening Outcomes: Comparing the Swedish and Finnish European Randomised Study of Screening for Prostate Cancer Centres
- PMID: 28803925
- PMCID: PMC5809235
- DOI: 10.1016/j.euf.2017.07.007
Impact of Prostatic-specific Antigen Threshold and Screening Interval in Prostate Cancer Screening Outcomes: Comparing the Swedish and Finnish European Randomised Study of Screening for Prostate Cancer Centres
Abstract
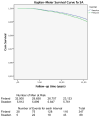
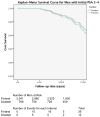
Background: The European Randomised Study of Screening for Prostate Cancer trial has shown a 21% reduction in prostate cancer (PC) mortality with prostate-specific antigen (PSA)-based screening. Sweden used a 2-yr screening interval and showed a larger mortality reduction than Finland with a 4-yr interval and higher PSA cut-off.
Objective: To evaluate the impact of screening interval and PSA cut-off on PC detection and mortality.
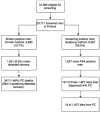
Design, setting, and participants: We analysed the core age groups (55-69 yr at entry) of the Finnish (N=31 866) and Swedish (N=5901) screening arms at 13 yr and 16 yr of follow-up. Sweden used a screening interval of 2 yr and a PSA cut-off of 3.0ng/ml, while in Finland the screening interval was 4 yr and the PSA cut-off 4.0ng/ml (or PSA 3.0-3.9ng/ml with free PSA<16%).
Outcome measurements and statistical analysis: We compared PC detection rate and PC mortality between the Finnish and Swedish centres and estimated the impact of different screening protocols.
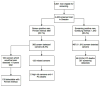
Results and limitations: If the Swedish screening protocol had been followed in Finland, 122 additional PC cases would have been diagnosed at screening, 84% of which would have been low-risk cancers, and four leading to PC death. In contrast, if a lower PSA threshold had been applied in Finland, at least 127 additional PC would have been found, with 19 PC deaths.
Conclusions: The small number of deaths among cases that would have been potentially detectable in Finland with the Swedish protocol (or those that would have been missed in Sweden with the Finnish approach) is unlikely to explain the differences in mortality in this long of a follow-up.
Patient summary: A prostate-specific antigen threshold of 3ng/ml versus 4ng/ml or a screening interval of 2 yr instead of 4 yr is unlikely to explain the larger mortality reduction achieved in Sweden compared with Finland.
Keywords: Prostate cancer; Prostate-specific antigen; Randomised trials; Screening.
Copyright © 2017 European Association of Urology. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest: None of the authors have conflicts of interest.
Figures
Similar articles
-
The Finnish prostate cancer screening trial: analyses on the screening failures.Int J Cancer. 2015 May 15;136(10):2437-43. doi: 10.1002/ijc.29300. Epub 2014 Nov 10. Int J Cancer. 2015. PMID: 25359457
-
Long-term Risk of Prostate Cancer Mortality Among Men with Baseline Prostate-specific Antigen Below 3 ng/ml: Evidence from the Finnish Randomized Study of Screening for Prostate Cancer.Eur Urol Oncol. 2025 Apr;8(2):452-459. doi: 10.1016/j.euo.2024.11.010. Epub 2024 Dec 6. Eur Urol Oncol. 2025. PMID: 39643505
-
Opportunistic testing versus organized prostate-specific antigen screening: outcome after 18 years in the Göteborg randomized population-based prostate cancer screening trial.Eur Urol. 2015 Sep;68(3):354-60. doi: 10.1016/j.eururo.2014.12.006. Epub 2014 Dec 31. Eur Urol. 2015. PMID: 25556937 Clinical Trial.
-
Prostate cancer screening with prostate-specific antigen (PSA) test: a systematic review and meta-analysis.BMJ. 2018 Sep 5;362:k3519. doi: 10.1136/bmj.k3519. BMJ. 2018. PMID: 30185521 Free PMC article.
-
Landmarks in prostate cancer screening.BJU Int. 2012 Oct;110 Suppl 1:3-7. doi: 10.1111/j.1464-410X.2012.011428.x. BJU Int. 2012. PMID: 23046034 Review.
Cited by
-
Exploring the Potential of MicroRNA Let-7c as a Therapeutic for Prostate Cancer.Mol Ther Nucleic Acids. 2019 Dec 6;18:927-937. doi: 10.1016/j.omtn.2019.09.031. Epub 2019 Oct 23. Mol Ther Nucleic Acids. 2019. PMID: 31760377 Free PMC article. Review.
-
The impact of different prostate-specific antigen (PSA) testing intervals on Gleason score at diagnosis and the risk of experiencing false-positive biopsy recommendations: a population-based cohort study.BMJ Open. 2019 Mar 30;9(3):e027958. doi: 10.1136/bmjopen-2018-027958. BMJ Open. 2019. PMID: 30928965 Free PMC article.
References
-
- Auvinen A, Moss Sm, Tammela Tl, Taari K, Roobol Mj, Schröder Fh, Bangma Ch, Carlsson S, Aus G, Zappa M, Puliti D, Denis Lj, Nelen V, Kwiatkowski M, Randazzo M, Paez A, Lujan M, Hugosson J. Absolute Effect Of Prostate Cancer Screening: Balance Of Benefits And Harms By Center Within The European Randomized Study Of Prostate Cancer Screening. Clinical Cancer Research. 2016;22:243–9. - PMC - PubMed
-
- Saarimaki L, Tammela Tl, Maattanen L, Taari K, Kujala Pm, Raitanen J, et al. Family History In The Finnish Prostate Cancer Screening Trial. International Journal Of Cancer. 2015;136:2172–7. - PubMed
-
- Greene Kl, Albertsen Pc, Babaian Rj, Carter Bh, Gann Ph, Han M, et al. Prostate Specific Antigen Best Practice Statement: 2009 Update. J Urol. 2013;189:S2–S11. - PubMed
-
- Heidenreich A, Bastian Pj, Bellmunt J, Bolla M, Joniau S, Van Der Kwast T, et al. EAU Guidelines On Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment With Curative Intent-Update 2013. Eur Urol. 2014;65:124–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

