The longitudinal effects of early developmental cadmium exposure on conditioned place preference and cardiovascular physiology in zebrafish
- PMID: 28804037
- PMCID: PMC5764186
- DOI: 10.1016/j.aquatox.2017.07.017
The longitudinal effects of early developmental cadmium exposure on conditioned place preference and cardiovascular physiology in zebrafish
Abstract
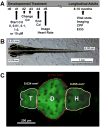
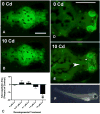
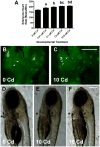
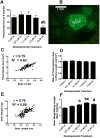
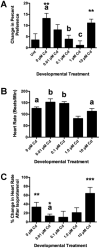
Cadmium (Cd) is a naturally occurring trace metal that is widely considered to be highly toxic to aquatic organisms and a significant health hazard to humans (Amzal et al., 2009; Bernhoft 2013; Burger, 2008; Satarug et al., 2009). The zebrafish (Danio rerio) has been used as a model organism for toxicological studies with Cd (Banni et al., 2011; Blechinger et al., 2007; Chow et al., 2009; Chow et al., 2008; Favorito et al., 2011; Kusch et al., 2007; Matz et al., 2007; Wang and Gallagher, 2013). We asked what the lasting longitudinal effects would be from short early developmental Cd exposure (between 24 and 96h post-fertilization) in a range that larvae might experience living atop typical Cd-containing surface sediments (0, 0.01, 0.1, 1.0 and 10μM CdCl2: 1.124, 11.24, 112.4 and 1124μg Cd/L). The goal of this exposure window was to specifically target secondary neurogenesis, monoaminergic differentiation and cardiovascular development, without affecting earlier patterning processes. Developmental abnormalities in body size and CNS morphology increased with concentration, but were statistically significant only at the highest concentration used (10μM). Heart rate for Cd-treated larvae increased with concentration, and was significant even at the lowest concentration used (0.01μM). Longitudinal survival was significantly lower for fish developmentally exposed to the highest concentration. Except for brain weight, overall morphology was not affected by developmental Cd exposure. However, developmental exposure to lower concentrations of Cd (0.01, 0.1, and 1.0μM) progressively lowered cocaine-induced conditioned place preference (CPP), used to measure function of the reward pathways in the brain. Baseline heart rate was significantly lower in longitudinal fish developmentally exposed to 1.0μM Cd. Cardiovascular response to isoproterenol, a potent ß-adrenergic agonist, in longitudinal adults was also significantly affected by developmental exposure to Cd at low doses (0.01, 0.1 and 1.0μM). Surviving longitudinal adult fish exposed to the highest concentration of Cd showed normal CPP and cardiovascular physiology. The data imply that even lower exposure concentrations can potentially result in fitness-affecting parameters without affecting survival in a laboratory setting.
Keywords: Behavior; Cadmium; Cardiovascular; Morphology; Zebrafish.
Copyright © 2017 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Longitudinal Effects of Embryonic Exposure to Cocaine on Morphology, Cardiovascular Physiology, and Behavior in Zebrafish.Int J Mol Sci. 2016 May 31;17(6):847. doi: 10.3390/ijms17060847. Int J Mol Sci. 2016. PMID: 27258254 Free PMC article.
-
Interaction of cadmium toxicity in embryos and larvae of zebrafish (Danio rerio) with calcium and humic substances.Aquat Toxicol. 2001 Oct;54(3-4):205-15. doi: 10.1016/s0166-445x(01)00145-x. Aquat Toxicol. 2001. PMID: 11489307
-
Effects of maternal cadmium exposure on female reproductive functions, gamete quality, and offspring development in zebrafish (Danio rerio).Arch Environ Contam Toxicol. 2013 Oct;65(3):521-36. doi: 10.1007/s00244-013-9909-1. Epub 2013 May 4. Arch Environ Contam Toxicol. 2013. PMID: 23644582
-
Cadmium significantly changes major morphometrical points and cardiovascular functional parameters during early development of zebrafish.Environ Toxicol Pharmacol. 2021 Oct;87:103723. doi: 10.1016/j.etap.2021.103723. Epub 2021 Aug 12. Environ Toxicol Pharmacol. 2021. PMID: 34391906
-
Effects of calcium and estrogen on the development of the ceratohyal cartilage in zebrafish (Danio rerio) larvae upon embryo and maternal cadmium exposure.Comp Biochem Physiol C Toxicol Pharmacol. 2018 Nov;213:47-54. doi: 10.1016/j.cbpc.2018.07.006. Epub 2018 Jul 27. Comp Biochem Physiol C Toxicol Pharmacol. 2018. PMID: 30059766
Cited by
-
Therapeutic Efficacy of Selenium Pre-treatment in Mitigating Cadmium-Induced Cardiotoxicity in Zebrafish (Danio rerio).Cardiovasc Toxicol. 2024 Nov;24(11):1287-1300. doi: 10.1007/s12012-024-09910-0. Epub 2024 Aug 30. Cardiovasc Toxicol. 2024. PMID: 39212842 Free PMC article.
-
Cardiac Development in the Presence of Cadmium: An in Vitro Study Using Human Embryonic Stem Cells and Cardiac Organoids.Environ Health Perspect. 2022 Nov;130(11):117002. doi: 10.1289/EHP11208. Epub 2022 Nov 2. Environ Health Perspect. 2022. PMID: 36321828 Free PMC article.
-
Therapeutic Efficacy of Selenium Pre-treatment in Mitigating Cadmium-Induced Cardiotoxicity in Zebrafish (Danio rerio).Res Sq [Preprint]. 2024 Jul 3:rs.3.rs-4583781. doi: 10.21203/rs.3.rs-4583781/v1. Res Sq. 2024. Update in: Cardiovasc Toxicol. 2024 Nov;24(11):1287-1300. doi: 10.1007/s12012-024-09910-0. PMID: 39011097 Free PMC article. Updated. Preprint.
-
Exposure to essential and non-essential trace elements and risks of congenital heart defects: A narrative review.Front Nutr. 2023 Mar 14;10:1121826. doi: 10.3389/fnut.2023.1121826. eCollection 2023. Front Nutr. 2023. PMID: 36998909 Free PMC article. Review.
-
Evaluation of cadmium and mercury on cardiovascular and neurological systems: Effects on humans and fish.Toxicol Rep. 2023 Apr 18;10:498-508. doi: 10.1016/j.toxrep.2023.04.009. eCollection 2023. Toxicol Rep. 2023. PMID: 37396852 Free PMC article. Review.
References
-
- Andres S, Ribeyre F, Tourencq JN, Boudou A. Interspecific comparison of cadmium and zinc contamination in the organs of four fish species along a polymetallic pollution gradient (Lot River, France) Science of the Total Environment. 2000;248:11–25. - PubMed
-
- Audry S, Schafer J, Blanc G, Jouanneau L. Fifty-year sedimentary record of heavy metal pollution (Cd, Zn, Cu, Pb) in the Lot River reservoirs (France) Environ Pollution. 2004;132:413–26. - PubMed
-
- Banni M, Chouchene L, Said K, Kerkeni A, Messaoudi I. Mechanisms underlying the protective effect of zinc and selenium against cadmium-induced oxidative stress in zebrafish. Biometals. 2011;24:981–92. - PubMed
-
- Barjhoux I, Gonzalez P, Baudrimont M, Cachot J. Molecular and phenotypic responses of Japanese medaka (Oryzias latipes) early life stages to environmental concentrations of cadmium in sediment. Environ Sci Pollut Res. 2016;23:17969–81. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

