Non-invasive urinary metabolomic profiling discriminates prostate cancer from benign prostatic hyperplasia
- PMID: 28804274
- PMCID: PMC5533870
- DOI: 10.1007/s11306-017-1194-y
Non-invasive urinary metabolomic profiling discriminates prostate cancer from benign prostatic hyperplasia
Abstract
Introduction: Prostate cancer (PCa) is one of the most common malignancies in men worldwide. Serum prostate specific antigen (PSA) level has been extensively used as a biomarker to detect PCa. However, PSA is not cancer-specific and various non-malignant conditions, including benign prostatic hyperplasia (BPH), can cause a rise in PSA blood levels, thus leading to many false positive results.
Objectives: In this study, we evaluated the potential of urinary metabolomic profiling for discriminating PCa from BPH.
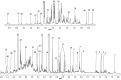
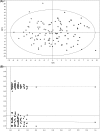
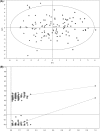
Methods: Urine samples from 64 PCa patients and 51 individuals diagnosed with BPH were analysed using 1H nuclear magnetic resonance (1H-NMR). Comparative analysis of urinary metabolomic profiles was carried out using multivariate and univariate statistical approaches.
Results: The urine metabolomic profile of PCa patients is characterised by increased concentrations of branched-chain amino acids (BCAA), glutamate and pseudouridine, and decreased concentrations of glycine, dimethylglycine, fumarate and 4-imidazole-acetate compared with individuals diagnosed with BPH.
Conclusion: PCa patients have a specific urinary metabolomic profile. The results of our study underscore the clinical potential of metabolomic profiling to uncover metabolic changes that could be useful to discriminate PCa from BPH in a clinical context.
Keywords: Benign prostatic hyperplasia; Biomarkers; Metabolomics; Nuclear magnetic resonance; Prostate cancer.
Conflict of interest statement
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki and applicable local regulatory requirements and laws and after approval from the Ethics Committee of the Instituto Valenciano de Oncología.
Ethical requirements
The manuscript is in compliance with ethical requirement of the journal.
Informed consent
Written informed consent was obtained from each participant before being included in this study.
Figures

References
-
- Andersen CM, Bro R. Variable selection in regression—a tutorial. Journal of Chemometrics. 2010;24(11-12):728–737. doi: 10.1002/cem.1360. - DOI
-
- Bianchi F, Dugheri S, Musci M, Bonacchi A, Salvadori E, Arcangeli G, et al. Fully automated solid-phase microextraction-fast gas chromatography-mass spectrometry method using a new ionic liquid column for high-throughput analysis of sarcosine and N-ethylglycine in human urine and urinary sediments. Analytica Chimica Acta. 2011;707(1–2):197–203. doi: 10.1016/j.aca.2011.09.015. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
