A Comprehensive Biophysical Model of Ion and Water Transport in Plant Roots. I. Clarifying the Roles of Endodermal Barriers in the Salt Stress Response
- PMID: 28804493
- PMCID: PMC5532442
- DOI: 10.3389/fpls.2017.01326
A Comprehensive Biophysical Model of Ion and Water Transport in Plant Roots. I. Clarifying the Roles of Endodermal Barriers in the Salt Stress Response
Abstract
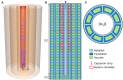
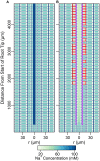
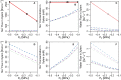
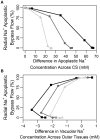
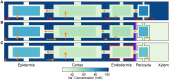
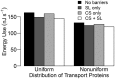
In this paper, we present a detailed and comprehensive mathematical model of active and passive ion and water transport in plant roots. Two key features are the explicit consideration of the separate, but interconnected, apoplastic, and symplastic transport pathways for ions and water, and the inclusion of both active and passive ion transport mechanisms. The model is used to investigate the respective roles of the endodermal Casparian strip and suberin lamellae in the salt stress response of plant roots. While it is thought that these barriers influence different transport pathways, it has proven difficult to distinguish their separate functions experimentally. In particular, the specific role of the suberin lamellae has been unclear. A key finding based on our simulations was that the Casparian strip is essential in preventing excessive uptake of Na+ into the plant via apoplastic bypass, with a barrier efficiency that is reflected by a sharp gradient in the steady-state radial distribution of apoplastic Na+ across the barrier. Even more significantly, this function cannot be replaced by the action of membrane transporters. The simulations also demonstrated that the positive effect of the Casparian strip of controlling Na+ uptake, was somewhat offset by its contribution to the osmotic stress component: a more effective barrier increased the detrimental osmotic stress effect. In contrast, the suberin lamellae were found to play a relatively minor, even non-essential, role in the overall response to salt stress, with the presence of the suberin lamellae resulting in only a slight reduction in Na+ uptake. However, perhaps more significantly, the simulations identified a possible role of suberin lamellae in reducing plant energy requirements by acting as a physical barrier to preventing the passive leakage of Na+ into endodermal cells. The model results suggest that more and particular experimental attention should be paid to the properties of the Casparian strip when assessing the salt tolerance of different plant varieties and species. Indeed, the Casparian strip appears to be a more promising target for plant breeding and plant genetic engineering efforts than the suberin lamellae for the goal of improving salt tolerance.
Keywords: Casparian strip; apoplastic and symplastic transport; osmotic stress; salt tolerance; suberin lamellae.
Figures



Similar articles
-
Role of LOTR1 in Nutrient Transport through Organization of Spatial Distribution of Root Endodermal Barriers.Curr Biol. 2017 Mar 6;27(5):758-765. doi: 10.1016/j.cub.2017.01.030. Epub 2017 Feb 23. Curr Biol. 2017. PMID: 28238658
-
The developmental dynamics of the sweet sorghum root transcriptome elucidate the differentiation of apoplastic barriers.Plant Signal Behav. 2020 Mar 3;15(3):1724465. doi: 10.1080/15592324.2020.1724465. Epub 2020 Feb 6. Plant Signal Behav. 2020. PMID: 32024414 Free PMC article.
-
Integrative physiology and transcriptome reveal salt-tolerance differences between two licorice species: Ion transport, Casparian strip formation and flavonoids biosynthesis.BMC Plant Biol. 2024 Apr 11;24(1):272. doi: 10.1186/s12870-024-04911-1. BMC Plant Biol. 2024. PMID: 38605293 Free PMC article.
-
Suberized transport barriers in plant roots: the effect of silicon.J Exp Bot. 2020 Dec 2;71(21):6799-6806. doi: 10.1093/jxb/eraa203. J Exp Bot. 2020. PMID: 32333766 Review.
-
The exodermis: a variable apoplastic barrier.J Exp Bot. 2001 Dec;52(365):2245-64. doi: 10.1093/jexbot/52.365.2245. J Exp Bot. 2001. PMID: 11709575 Review.
Cited by
-
A root functional-structural model allows assessment of the effects of water deficit on water and solute transport parameters.J Exp Bot. 2023 Mar 13;74(5):1594-1608. doi: 10.1093/jxb/erac471. J Exp Bot. 2023. PMID: 36515073 Free PMC article.
-
A Comprehensive Biophysical Model of Ion and Water Transport in Plant Roots. III. Quantifying the Energy Costs of Ion Transport in Salt-Stressed Roots of Arabidopsis.Front Plant Sci. 2020 Jul 3;11:865. doi: 10.3389/fpls.2020.00865. eCollection 2020. Front Plant Sci. 2020. PMID: 32719693 Free PMC article.
-
A Comprehensive Biophysical Model of Ion and Water Transport in Plant Roots. II. Clarifying the Roles of SOS1 in the Salt-Stress Response in Arabidopsis.Front Plant Sci. 2019 Sep 18;10:1121. doi: 10.3389/fpls.2019.01121. eCollection 2019. Front Plant Sci. 2019. PMID: 31620152 Free PMC article.
-
The influences of stomatal size and density on rice abiotic stress resilience.New Phytol. 2023 Mar;237(6):2180-2195. doi: 10.1111/nph.18704. Epub 2023 Jan 11. New Phytol. 2023. PMID: 36630602 Free PMC article.
-
Barriers and carriers for transition metal homeostasis in plants.Plant Commun. 2025 Feb 10;6(2):101235. doi: 10.1016/j.xplc.2024.101235. Epub 2024 Dec 26. Plant Commun. 2025. PMID: 39731291 Free PMC article. Review.
References
-
- Anil V. S., Krishnamurthy H., Mathew M. K. (2007). Limiting cytosolic Na+ confers salt tolerance to rice cells in culture: a two-photon microscopy study of SBFI-loaded cells. Physiol. Plant. 129, 607–621. 10.1111/j.1399-3054.2006.00854.x - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

