Treatment of older patients with acute myeloid leukemia (AML): revised Canadian consensus guidelines
- PMID: 28804680
- PMCID: PMC5545212
Treatment of older patients with acute myeloid leukemia (AML): revised Canadian consensus guidelines
Abstract
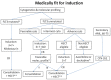
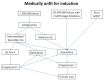
The treatment of acute myeloid leukemia (AML) in older patients is undergoing rapid changes, with a number of important publications in the past five years. Because of this, a group of Canadian leukemia experts has produced an update to the Canadian Consensus Guidelines that were published in 2013, with several new agents recommended, subject to availability. Recent studies have supported the survival benefit of induction chemotherapy for patients under age 80, except those with major co-morbidities or those with adverse risk cytogenetics who are not candidates for allogeneic hematopoietic stem cell transplantation (HSCT). Midostaurin should be added to induction therapy for patients up to age 70 with a FLT3 mutation, and gemtuzumab ozogamicin for de novo AML up to age 70 with favorable or intermediate risk cytogenetics. Daunorubicin 60 mg/m2 is the recommended dose for 3+7 induction therapy. Acute promyelocytic leukemia should be treated with arsenic trioxide plus all-trans retinoic acid, regardless of age, with cytotoxic therapy added upfront only for those with initial white blood count > 10. HSCT may be considered for selected suitable patients up to age 70-75. Haploidentical donor transplants may be considered for older patients. For non-induction candidates, azacitidine is recommended for those with adverse risk cytogenetics, while either a hypomethylating agent (HMA) or low-dose cytarabine can be used for others. HMA may also be used for relapsed/refractory disease after chemotherapy. For patients with secondary AML, CPX-351 is recommended for fit patients age 60-75.
Keywords: Acute myeloid leukemia; chemotherapy; co-morbidities; cytogenetics; elderly patients; hematopoietic stem cell transplantation; hypomethylating agents.
Conflict of interest statement
JM Brandwein: Advisory Board & Honoraria - Celgene, Lundbeck, Pfizer, Novartis, Paladin. N Zhu: Advisory Board & Honoraria - Celgene, Novartis. R Kumar: Advisory Board & Honoraria - Celgene. B Leber: Advisory Board and Honoraria - Celgene, Novartis, BMS, Pfizer, Paladin, Amgen, Alexion, Lundbeck, Abbvie, Astellas. M Sabloff: Lundbeck, Amgen. Research funding - Sanofi, Roche. I Sandhu: Advisory Board & Honoraria - Celgene, Novartis. J Kassis: No disclosures. HJ Olney: Advisory Board & Honoraria - Celgene, BMS, Novartis, Paladin, Pfizer. M Elemary: Advisory Board & Honoraria - Celgene, Novartis, Roche, Lundbeck. AC Schuh: Advisory Board & Honoraria - Celgene, Lundbeck, Pfizer.
Figures
References
-
- Sorror ML, Storer BE, Elsawy M, Fathi AT, Brunner A, Gerds AT, Sekeres MA, Mukherjee S, Medeiros BC, Shami P, Peña E, Wardyn S, Whitten J, Frenkel H, McCune J, Lee SJ, Estey EH. Impact of comorbidities at diagnosis of acute myeloid leukemia on one-year mortality. Blood. 2015;126:532.
-
- Sorror ML, Storer BE, Elsawy M, Fathi AT, Brunner A, Gerds AT, Sekeres MA, Mukherjee S, Medeiros BC, Wang ES, Vachhani P, Shami PJ, Peña E, Wardyn S, Whitten J, Moore R, Becker PS, McCune J, Lee SJ, Sandmaier BM, Appelbaum FR, Estey EH. Intensive versus non-intensive induction therapy for patients (Pts) with newly diagnosed acute myeloid leukemia (AML) using two different novel prognostic models. Blood. 2016;128:216.
-
- Juliusson G, Antunovic P, Derolf A, Lehmann S, Möllgård L, Stockelberg D, Tidefelt U, Wahlin A, Höglund M. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish acute leukemia registry. Blood. 2009;113:4179–4187. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
