Bioresorbable scaffold as a dermal substitute
- PMID: 28804684
- PMCID: PMC5545429
Bioresorbable scaffold as a dermal substitute
Abstract
Introduction: Bioresorbable polymers are often used in medical procedures. Since they are biocompatible, this class of materials is a viable alternative for cases in which tissue regeneration is strongly compromised. Bioresorbable synthetic polymers may be used as membranes to support and guide cell growth through the process of tissue repair.
Objective: To assess the efficiency of a porous bioresorbable membrane Poly (L-co-DL lactic acid)-co-trimethylene carbonate, PL-co-DLA-co-TMC, as a dermal substitute associated with partial skin graft in rats.
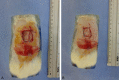
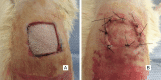
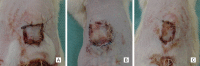
Methods: A 1.5×1.5 cm defect was created on the backs of 40 Wistar rats. The rats were divided into a control group, in which the defects were filled with partial skin graft, and a treated group, in which a membrane associated with the graft was implemented. The animals were sacrificed 7 days or 60 days after the procedure and the results were evaluated by macroscopic and microscopic analysis.
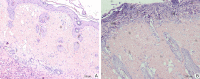
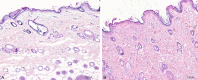
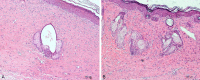
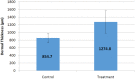
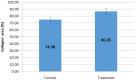
Results: The polymer was biocompatible and allowed better regeneration of the dermis with less shrinkage, unlike what occurs in second intention healing. Compared to the control group, the treated group showed a thicker and wider dermis with the presence of skin appendages, suggesting partial graft integration and better healing. The skin graft acted as a biological protection of the wound.
Conclusion: The study material was shown to act as a biocompatible dermal substitute and promoted less scarring of the dermis. Further studies should be conducted to improve the methodology of the surgical procedure.
Keywords: Cutaneous wound; bioresorbable polymer; dermal substitute; graft; in vivo study.
Conflict of interest statement
None.
Figures













References
-
- Ferreira MC, Paggiaro AO, Isaac C, Teixeira Neto N, Santos GB. Substitutos cutâneos: conceitos atuais e proposta de classificação. Rev Bras Cir Plást. 2011;26:696–702.
-
- Ferreira MC, Tuma P Jr, Carvalho VF, Kamamoto F. Complex wounds. Clinics (São Paulo) 2006;61:571–8. - PubMed
-
- Leung JJ, Fish J. Skin Grafts. UTMJ. 2009;86:61–4.
-
- Wainwright DJ, Bury SB. Acellular dermal matrix in the management of the burn patient. Aesthet Surg J. 2011;31(Suppl):13S–23S. - PubMed
LinkOut - more resources
Full Text Sources
