Meprin Metalloprotease Deficiency Associated with Higher Mortality Rates and More Severe Diabetic Kidney Injury in Mice with STZ-Induced Type 1 Diabetes
- PMID: 28804725
- PMCID: PMC5540529
- DOI: 10.1155/2017/9035038
Meprin Metalloprotease Deficiency Associated with Higher Mortality Rates and More Severe Diabetic Kidney Injury in Mice with STZ-Induced Type 1 Diabetes
Erratum in
-
Erratum to "Meprin Metalloprotease Deficiency Associated with Higher Mortality Rates and More Severe Diabetic Kidney Injury in Mice with STZ-Induced Type 1 Diabetes".J Diabetes Res. 2018 Jul 5;2018:2462697. doi: 10.1155/2018/2462697. eCollection 2018. J Diabetes Res. 2018. PMID: 30116735 Free PMC article.
Abstract
Meprins are membrane-bound and secreted metalloproteinases consisting of α and/or β subunits that are highly expressed in kidney epithelial cells and are differentially expressed in podocytes and leukocytes (macrophages and monocytes). Several studies have implicated meprins in the progression of diabetic nephropathy (DN) and fibrosis-associated kidney disease. However, the mechanisms by which meprins modulate DN are not understood. To delineate the role of meprins in DN, we subjected meprin αβ knockout (αβKO) mice and their wild-type (WT) counterparts to streptozotocin-induced type 1 diabetes. The 18-week survival rates were significantly lower for diabetic meprin αβKO mice when compared to those for their WT counterparts. There were significant decreases in mRNA and protein levels for both meprin α and β in diabetic WT kidneys. Furthermore, the blood urea nitrogen levels and urine albumin/creatinine ratios increased in diabetic meprin αβKO but not in diabetic WT mice, indicating that meprins may be protective against diabetic kidney injury. The brush border membrane levels of villin, a meprin target, significantly decreased in diabetic WT but not in diabetic meprin αβKO kidneys. In contrast, isoform-specific increases in cytosolic levels of the catalytic subunit of PKA, another meprin target, were demonstrated for both WT and meprin αβKO kidneys.
Figures

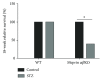



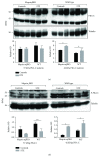

Similar articles
-
Meprin β metalloproteases associated with differential metabolite profiles in the plasma and urine of mice with type 1 diabetes and diabetic nephropathy.BMC Nephrol. 2019 Apr 25;20(1):141. doi: 10.1186/s12882-019-1313-2. BMC Nephrol. 2019. PMID: 31023251 Free PMC article.
-
Isoform-specific interactions between meprin metalloproteases and the catalytic subunit of protein kinase A: significance in acute and chronic kidney injury.Am J Physiol Renal Physiol. 2015 Jan 1;308(1):F56-68. doi: 10.1152/ajprenal.00167.2014. Epub 2014 Oct 29. Am J Physiol Renal Physiol. 2015. PMID: 25354939 Free PMC article.
-
LC-MS-based metabolomics analysis to identify meprin-β-associated changes in kidney tissue from mice with STZ-induced type 1 diabetes and diabetic kidney injury.Am J Physiol Renal Physiol. 2019 Oct 1;317(4):F1034-F1046. doi: 10.1152/ajprenal.00166.2019. Epub 2019 Aug 14. Am J Physiol Renal Physiol. 2019. PMID: 31411076 Free PMC article.
-
Role of meprin metalloproteinases in cytokine processing and inflammation.Cytokine. 2019 Feb;114:18-25. doi: 10.1016/j.cyto.2018.11.032. Epub 2018 Dec 20. Cytokine. 2019. PMID: 30580156 Free PMC article. Review.
-
Meprin A metalloproteinase and its role in acute kidney injury.Am J Physiol Renal Physiol. 2013 May 1;304(9):F1150-8. doi: 10.1152/ajprenal.00014.2013. Epub 2013 Feb 20. Am J Physiol Renal Physiol. 2013. PMID: 23427141 Free PMC article. Review.
Cited by
-
Exploring Computational Data Amplification and Imputation for the Discovery of Type 1 Diabetes (T1D) Biomarkers from Limited Human Datasets.Biomolecules. 2022 Oct 9;12(10):1444. doi: 10.3390/biom12101444. Biomolecules. 2022. PMID: 36291653 Free PMC article.
-
Undiagnosed Kidney Injury in Uninsured and Underinsured Diabetic African American Men and Putative Role of Meprin Metalloproteases in Diabetic Nephropathy.Int J Nephrol. 2018 Apr 29;2018:6753489. doi: 10.1155/2018/6753489. eCollection 2018. Int J Nephrol. 2018. PMID: 29854459 Free PMC article.
-
Reduced Expression of Extracellular Matrix Proteins in the Heart and Kidneys of Rats with Endotoxemia under the Effect of Actinonin.Bull Exp Biol Med. 2021 Apr;170(6):744-747. doi: 10.1007/s10517-021-05146-y. Epub 2021 Apr 24. Bull Exp Biol Med. 2021. PMID: 33893962
-
Meprin-β activity modulates the β-catalytic subunit of protein kinase A in ischemia-reperfusion-induced acute kidney injury.Am J Physiol Renal Physiol. 2020 May 1;318(5):F1147-F1159. doi: 10.1152/ajprenal.00571.2019. Epub 2020 Mar 16. Am J Physiol Renal Physiol. 2020. PMID: 32174142 Free PMC article.
-
Erratum to "Meprin Metalloprotease Deficiency Associated with Higher Mortality Rates and More Severe Diabetic Kidney Injury in Mice with STZ-Induced Type 1 Diabetes".J Diabetes Res. 2018 Jul 5;2018:2462697. doi: 10.1155/2018/2462697. eCollection 2018. J Diabetes Res. 2018. PMID: 30116735 Free PMC article.
References
-
- Beynon R. J., Bond J. S. Expression of the Mep-1 gene regulating meprin, a kidney brush border proteinase. 1985;180:185–194. - PubMed
-
- Becker-Pauly C., Howel M., Walker T., et al. The alpha and beta subunits of the metalloprotease meprin are expressed in separate layers of human epidermis, revealing different functions in keratinocyte proliferation and differentiation. 2007;127(5):1115–1125. doi: 10.1038/sj.jid.5700675. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

