Probing biological redox chemistry with large amplitude Fourier transformed ac voltammetry
- PMID: 28804798
- PMCID: PMC5708363
- DOI: 10.1039/c7cc03870d
Probing biological redox chemistry with large amplitude Fourier transformed ac voltammetry
Abstract
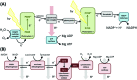
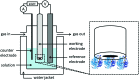
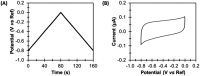
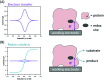
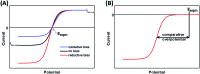
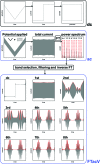
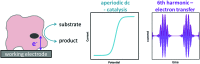
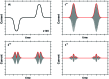
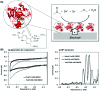
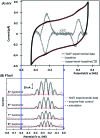
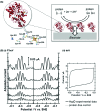
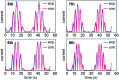
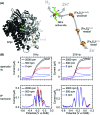
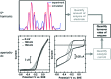
Biological electron-exchange reactions are fundamental to life on earth. Redox reactions underpin respiration, photosynthesis, molecular biosynthesis, cell signalling and protein folding. Chemical, biomedical and future energy technology developments are also inspired by these natural electron transfer processes. Further developments in techniques and data analysis are required to gain a deeper understanding of the redox biochemistry processes that power Nature. This review outlines the new insights gained from developing Fourier transformed ac voltammetry as a tool for protein film electrochemistry.
Figures

Similar articles
-
Investigation of mediated oxidation of ascorbic acid by ferrocenemethanol using large-amplitude Fourier transformed ac voltammetry under quasi-reversible electron-transfer conditions at an indium tin oxide electrode.Anal Chem. 2008 Sep 1;80(17):6515-25. doi: 10.1021/ac702531f. Epub 2008 Jul 31. Anal Chem. 2008. PMID: 18666783
-
Detailed analysis of the electron-transfer properties of azurin adsorbed on graphite electrodes using DC and large-amplitude Fourier transformed AC voltammetry.Anal Chem. 2007 Sep 1;79(17):6515-26. doi: 10.1021/ac070448j. Epub 2007 Aug 2. Anal Chem. 2007. PMID: 17668927
-
Large amplitude Fourier transformed AC voltammetric investigation of the active state electrochemistry of a copper/aqueous base interface and implications for electrocatalysis.Langmuir. 2011 Aug 16;27(16):10302-11. doi: 10.1021/la2017819. Epub 2011 Jul 20. Langmuir. 2011. PMID: 21721544
-
Recent advances in the electrochemistry and spectroelectrochemistry of membrane proteins.Biol Chem. 2013 May;394(5):593-609. doi: 10.1515/hsz-2012-0344. Biol Chem. 2013. PMID: 23362196 Review.
-
Concerted proton-electron transfers: fundamentals and recent developments.Annu Rev Anal Chem (Palo Alto Calif). 2014;7:537-60. doi: 10.1146/annurev-anchem-071213-020315. Annu Rev Anal Chem (Palo Alto Calif). 2014. PMID: 25014349 Review.
Cited by
-
Enzymatic and Microbial Electrochemistry: Approaches and Methods.ACS Meas Sci Au. 2022 Dec 21;2(6):517-541. doi: 10.1021/acsmeasuresciau.2c00042. Epub 2022 Aug 29. ACS Meas Sci Au. 2022. PMID: 36573075 Free PMC article. Review.
-
Conserved Histidine Adjacent to the Proximal Cluster Tunes the Anaerobic Reductive Activation of Escherichia coli Membrane-Bound [NiFe] Hydrogenase-1.ChemElectroChem. 2018 Mar;5(6):855-860. doi: 10.1002/celc.201800047. Epub 2018 Feb 16. ChemElectroChem. 2018. PMID: 29696103 Free PMC article.
-
Redox Electrochemistry to Interrogate and Control Biomolecular Communication.iScience. 2020 Sep 8;23(9):101545. doi: 10.1016/j.isci.2020.101545. eCollection 2020 Sep 25. iScience. 2020. PMID: 33083771 Free PMC article. Review.
-
A Voltammetric Perspective of Multi-Electron and Proton Transfer in Protein Redox Chemistry: Insights From Computational Analysis of Escherichia coli HypD Fourier Transformed Alternating Current Voltammetry.Front Chem. 2021 Jun 14;9:672831. doi: 10.3389/fchem.2021.672831. eCollection 2021. Front Chem. 2021. PMID: 34195174 Free PMC article.
-
Applying Nuclear Forward Scattering as In Situ and Operando Tool for the Characterization of FeN4 Moieties in the Hydrogen Evolution Reaction.J Am Chem Soc. 2024 May 8;146(18):12496-12510. doi: 10.1021/jacs.4c00436. Epub 2024 Apr 17. J Am Chem Soc. 2024. PMID: 38630640 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

