Chronic Ethanol Metabolism Inhibits Hepatic Mitochondrial Superoxide Dismutase via Lysine Acetylation
- PMID: 28804911
- PMCID: PMC5626652
- DOI: 10.1111/acer.13473
Chronic Ethanol Metabolism Inhibits Hepatic Mitochondrial Superoxide Dismutase via Lysine Acetylation
Abstract
Background: Chronic ethanol (EtOH) consumption is a major cause of liver disease worldwide. Oxidative stress is a known consequence of EtOH metabolism and is thought to contribute significantly to alcoholic liver disease (ALD). Therefore, elucidating pathways leading to sustained oxidative stress and downstream redox imbalances may reveal how EtOH consumption leads to ALD. Recent studies suggest that EtOH metabolism impacts mitochondrial antioxidant processes through a number of proteomic alterations, including hyperacetylation of key antioxidant proteins.
Methods: To elucidate mechanisms of EtOH-induced hepatic oxidative stress, we investigate a role for protein hyperacetylation in modulating mitochondrial superoxide dismutase (SOD2) structure and function in a 6-week Lieber-DeCarli murine model of EtOH consumption. Our experimental approach includes immunoblotting immunohistochemistry (IHC), activity assays, mass spectrometry, and in silico modeling.
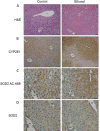
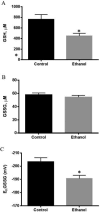
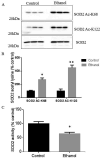
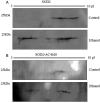
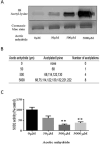
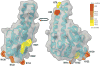
Results: We found that EtOH metabolism significantly increased the acetylation of SOD2 at 2 functionally relevant lysine sites, K68 and K122, resulting in a 40% decrease in enzyme activity while overall SOD2 abundance was unchanged. In vitro studies also reveal which lysine residues are more susceptible to acetylation. IHC analysis demonstrates that SOD2 hyperacetylation occurs near zone 3 within the liver, which is the main EtOH-metabolizing region of the liver.
Conclusions: Overall, the findings presented in this study support a role for EtOH-induced lysine acetylation as an adverse posttranslational modification within the mitochondria that directly impacts SOD2 charge state and activity. Last, the data presented here indicate that protein hyperacetylation may be a major factor contributing to an imbalance in hepatic redox homeostasis due to chronic EtOH metabolism.
Keywords: Alcoholic Liver Disease; Lysine Acetylation; Oxidative Stress; Sirtuin; Superoxide Dismutase.
Copyright © 2017 by the Research Society on Alcoholism.
Conflict of interest statement
The authors have no conflict of interest to disclose.
Figures
Similar articles
-
Chronic ethanol consumption induces mitochondrial protein acetylation and oxidative stress in the kidney.Redox Biol. 2015 Dec;6:33-40. doi: 10.1016/j.redox.2015.06.021. Epub 2015 Jul 6. Redox Biol. 2015. PMID: 26177469 Free PMC article.
-
Alcohol- and Low-Iron Induced Changes in Antioxidant and Energy Metabolism Associated with Protein Lys Acetylation.Int J Mol Sci. 2024 Jul 30;25(15):8344. doi: 10.3390/ijms25158344. Int J Mol Sci. 2024. PMID: 39125916 Free PMC article.
-
In Vivo Metabolic Tracing Demonstrates the Site-Specific Contribution of Hepatic Ethanol Metabolism to Histone Acetylation.Alcohol Clin Exp Res. 2018 Oct;42(10):1909-1923. doi: 10.1111/acer.13843. Epub 2018 Aug 16. Alcohol Clin Exp Res. 2018. PMID: 30030934 Free PMC article.
-
Alcohol-induced protein hyperacetylation: mechanisms and consequences.World J Gastroenterol. 2009 Mar 14;15(10):1219-30. doi: 10.3748/wjg.15.1219. World J Gastroenterol. 2009. PMID: 19291822 Free PMC article. Review.
-
A Unifying Hypothesis Linking Hepatic Adaptations for Ethanol Metabolism to the Proinflammatory and Profibrotic Events of Alcoholic Liver Disease.Alcohol Clin Exp Res. 2018 Nov;42(11):2072-2089. doi: 10.1111/acer.13877. Epub 2018 Sep 17. Alcohol Clin Exp Res. 2018. PMID: 30132924 Free PMC article. Review.
Cited by
-
Mitochondrial fusion and Bid-mediated mitochondrial apoptosis are perturbed by alcohol with distinct dependence on its metabolism.Cell Death Dis. 2018 Oct 9;9(10):1028. doi: 10.1038/s41419-018-1070-3. Cell Death Dis. 2018. PMID: 30301883 Free PMC article.
-
Neuronal SIRT3 Deletion Predisposes to Female-Specific Alterations in Cellular Metabolism, Memory, and Network Excitability.J Neurosci. 2023 Mar 8;43(10):1845-1857. doi: 10.1523/JNEUROSCI.1259-22.2023. Epub 2023 Feb 9. J Neurosci. 2023. PMID: 36759193 Free PMC article.
-
Ethanol Intoxication Impairs Respiratory Function and Bacterial Clearance and Is Associated With Neutrophil Accumulation in the Lung After Streptococcus pneumoniae Infection.Front Immunol. 2022 May 4;13:884719. doi: 10.3389/fimmu.2022.884719. eCollection 2022. Front Immunol. 2022. PMID: 35603143 Free PMC article.
-
Alcohol-Related Liver Disease: An Overview on Pathophysiology, Diagnosis and Therapeutic Perspectives.Biomedicines. 2022 Oct 10;10(10):2530. doi: 10.3390/biomedicines10102530. Biomedicines. 2022. PMID: 36289791 Free PMC article. Review.
-
Cholestatic liver disease results increased production of reactive aldehydes and an atypical periportal hepatic antioxidant response.Free Radic Biol Med. 2019 Nov 1;143:101-114. doi: 10.1016/j.freeradbiomed.2019.07.036. Epub 2019 Aug 1. Free Radic Biol Med. 2019. PMID: 31377417 Free PMC article.
References
-
- Armstrong JS, Whiteman M, Yang H, Jones DP. The redox regulation of intermediary metabolism by a superoxide-aconitase rheostat. Bioessays. 2004;26:894–900. - PubMed
-
- CDC. Average for United States 2006–2010 Alcohol-Attributable Deaths Due to Excessive Alcohol Use. Centers for Disease Control and Prevention (CDC); 2013. Alcohol and Public Health: Alcohol-Related Disease Impact (ARDI)
-
- Cederbaum AI, Lu Y, Wu D. Role of oxidative stress in alcohol-induced liver injury. Arch Toxicol. 2009;83:519–48. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

