Wiskott-Aldrich syndrome protein: Emerging mechanisms in immunity
- PMID: 28805251
- PMCID: PMC7612068
- DOI: 10.1002/eji.201646715
Wiskott-Aldrich syndrome protein: Emerging mechanisms in immunity
Abstract
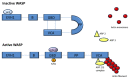
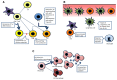
The Wiskott-Aldrich syndrome protein (WASP) participates in innate and adaptive immunity through regulation of actin cytoskeleton-dependent cellular processes, including immune synapse formation, cell signaling, migration and cytokine release. There is also emerging evidence for a direct role in nuclear transcription programmes uncoupled from actin polymerization. A deeper understanding of some of the more complex features of Wiskott Aldrich syndrome (WAS) itself, such as the associated autoimmunity and inflammation, has come from identification of defects in the number and function of anti-inflammatory myeloid cells and regulatory T and B cells, as well as defects in positive and negative B-cell selection. In this review we outline the cellular defects that have been characterized in both human WAS patients and murine models of the disease. We will emphasize in particular recent discoveries that provide a mechanistic insight into disease pathology, including lymphoid and myeloid cell homeostasis, immune synapse assembly and immune cell signaling.
Keywords: Autoimmunity; Immune synapse; Inflammation; Wiskott Aldrich syndrome; Wiskott Aldrich syndrome protein.
© 2017 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
Figures
References
-
- Derry JM, Ochs HD, Francke U. Isolation of a novel gene mutated in Wiskott-Aldrich syndrome. Cell. 1994;78:635–644. - PubMed
-
- Worth AJ, Thrasher AJ. Current and emerging treatment options for Wiskott-Aldrich syndrome. Expert Rev Clin Immunol. 2015;11:1015–1032. - PubMed
-
- Kim AS, Kakalis LT, Abdul-Manan N, Liu GA, Rosen MK. Autoinhibition and activation mechanisms of the Wiskott-Aldrich syndrome protein. Nature. 2000;404:151–158. - PubMed
-
- Panchal SC, Kaiser DA, Torres E, Pollard TD, Rosen MK. A conserved amphipathic helix in WASP/Scar proteins is essential for activation of Arp2/3 complex. Nat Struct Biol. 2003;10:591–598. - PubMed
-
- Cory GO, Garg R, Cramer R, Ridley AJ. Phosphorylation of tyrosine 291 enhances the ability of WASp to stimulate actin polymerization and filopodium formation. Wiskott-Aldrich Syndrome protein. J Biol Chem. 2002;277:45115–45121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

