Bioprocess scale-up/down as integrative enabling technology: from fluid mechanics to systems biology and beyond
- PMID: 28805306
- PMCID: PMC5609235
- DOI: 10.1111/1751-7915.12803
Bioprocess scale-up/down as integrative enabling technology: from fluid mechanics to systems biology and beyond
Abstract
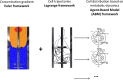
Efficient optimization of microbial processes is a critical issue for achieving a number of sustainable development goals, considering the impact of microbial biotechnology in agrofood, environment, biopharmaceutical and chemical industries. Many of these applications require scale-up after proof of concept. However, the behaviour of microbial systems remains unpredictable (at least partially) when shifting from laboratory-scale to industrial conditions. The need for robust microbial systems is thus highly needed in this context, as well as a better understanding of the interactions between fluid mechanics and cell physiology. For that purpose, a full scale-up/down computational framework is already available. This framework links computational fluid dynamics (CFD), metabolic flux analysis and agent-based modelling (ABM) for a better understanding of the cell lifelines in a heterogeneous environment. Ultimately, this framework can be used for the design of scale-down simulators and/or metabolically engineered cells able to cope with environmental fluctuations typically found in large-scale bioreactors. However, this framework still needs some refinements, such as a better integration of gas-liquid flows in CFD, and taking into account intrinsic biological noise in ABM.
© 2017 The Authors. Microbial Biotechnology published by John Wiley & Sons Ltd and Society for Applied Microbiology.
Figures


Similar articles
-
Metabolic variability in bioprocessing: implications of microbial phenotypic heterogeneity.Trends Biotechnol. 2014 Dec;32(12):608-16. doi: 10.1016/j.tibtech.2014.10.002. Epub 2014 Oct 22. Trends Biotechnol. 2014. PMID: 25457387
-
Coupled metabolic-hydrodynamic modeling enabling rational scale-up of industrial bioprocesses.Biotechnol Bioeng. 2020 Mar;117(3):844-867. doi: 10.1002/bit.27243. Epub 2019 Dec 20. Biotechnol Bioeng. 2020. PMID: 31814101 Review.
-
Bioreactors and bioseparation.Adv Biochem Eng Biotechnol. 2010;122:105-50. doi: 10.1007/10_2010_70. Adv Biochem Eng Biotechnol. 2010. PMID: 20396995
-
[Improving industrial microbial stress resistance by metabolic engineering: a review].Sheng Wu Gong Cheng Xue Bao. 2010 Sep;26(9):1209-17. Sheng Wu Gong Cheng Xue Bao. 2010. PMID: 21141110 Review. Chinese.
-
Application of systems biology for bioprocess development.Trends Biotechnol. 2008 Aug;26(8):404-12. doi: 10.1016/j.tibtech.2008.05.001. Epub 2008 Jun 24. Trends Biotechnol. 2008. PMID: 18582974 Review.
Cited by
-
In Silico Prediction of Large-Scale Microbial Production Performance: Constraints for Getting Proper Data-Driven Models.Comput Struct Biotechnol J. 2018 Jul 6;16:246-256. doi: 10.1016/j.csbj.2018.06.002. eCollection 2018. Comput Struct Biotechnol J. 2018. PMID: 30105090 Free PMC article. Review.
-
Monitoring Intracellular Metabolite Dynamics in Saccharomyces cerevisiae during Industrially Relevant Famine Stimuli.Metabolites. 2022 Mar 18;12(3):263. doi: 10.3390/metabo12030263. Metabolites. 2022. PMID: 35323706 Free PMC article.
-
Automated Conditional Screening of Multiple Escherichia coli Strains in Parallel Adaptive Fed-Batch Cultivations.Bioengineering (Basel). 2020 Nov 11;7(4):145. doi: 10.3390/bioengineering7040145. Bioengineering (Basel). 2020. PMID: 33187191 Free PMC article.
-
Deep in situ microscopy for real-time analysis of mammalian cell populations in bioreactors.Sci Rep. 2023 Dec 12;13(1):22045. doi: 10.1038/s41598-023-48733-x. Sci Rep. 2023. PMID: 38086908 Free PMC article.
-
A validated strategy to design efficient fermentation-industrial processes: agave spirit production.Bioprocess Biosyst Eng. 2021 Nov;44(11):2245-2255. doi: 10.1007/s00449-021-02600-z. Epub 2021 Jun 22. Bioprocess Biosyst Eng. 2021. PMID: 34156516
References
-
- Aldor, I. S. , Krawitz, D. C. , Forrest, W. , Chen, C. , Nishihara, J. C. , Joly, J. C. , et al (2005) Proteomic profiling of recombinant Escherichia coli in high‐cell‐density fermentations for improved production of an antibody fragment biopharmaceutical. Appl Environ Microbiol 71: 1717–1728. - PMC - PubMed
-
- Baert, J. , Kinet, R. , Brognaux, A. , Delepierre, A. , Telek, S. , Sorensen, S. J. , et al (2015) Phenotypic variability in bioprocessing conditions can be tracked on the basis of on‐line flow cytometry and fits to a scaling law. Biotechnol J 10: 1316–1325. - PubMed
-
- Binder, D. , Drepper, T. , Jaeger, K.‐E. , Delvigne, F. , Wiechert, W. , Kohlheyer, D. , et al (2017) Homogenizing bacterial cell factories: analysis and engineering of phenotypic heterogeneity. Metab Eng 42: 145–156. - PubMed
-
- Boer, V. M. , de Winde, J. H. , Pronk, J. T. , and Piper, M. D. W. (2003) The genome‐wide transcriptional responses of Saccharomyces cerevisiae grown on glucose in aerobic chemostat cultures limited for carbon, nitrogen, phosphorus, or sulfur. J Biol Chem 278: 3265–3274. - PubMed
-
- Broger, T. , Odermatt, R. P. , Huber, P. , and Sonnleitner, B. (2011) Real‐time on‐line flow cytometry for bioprocess monitoring. J Biotechnol 154: 240–247. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

