Opposing effects of cancer-type-specific SPOP mutants on BET protein degradation and sensitivity to BET inhibitors
- PMID: 28805821
- PMCID: PMC5592092
- DOI: 10.1038/nm.4372
Opposing effects of cancer-type-specific SPOP mutants on BET protein degradation and sensitivity to BET inhibitors
Abstract
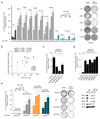
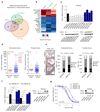
It is generally assumed that recurrent mutations within a given cancer driver gene elicit similar drug responses. Cancer genome studies have identified recurrent but divergent missense mutations affecting the substrate-recognition domain of the ubiquitin ligase adaptor SPOP in endometrial and prostate cancers. The therapeutic implications of these mutations remain incompletely understood. Here we analyzed changes in the ubiquitin landscape induced by endometrial cancer-associated SPOP mutations and identified BRD2, BRD3 and BRD4 proteins (BETs) as SPOP-CUL3 substrates that are preferentially degraded by endometrial cancer-associated SPOP mutants. The resulting reduction of BET protein levels sensitized cancer cells to BET inhibitors. Conversely, prostate cancer-specific SPOP mutations resulted in impaired degradation of BETs, promoting their resistance to pharmacologic inhibition. These results uncover an oncogenomics paradox, whereby mutations mapping to the same domain evoke opposing drug susceptibilities. Specifically, we provide a molecular rationale for the use of BET inhibitors to treat patients with endometrial but not prostate cancer who harbor SPOP mutations.
Conflict of interest statement
Competing Financial Interest
L.A.G is a paid consultant of the following pharmaceutical companies: Novartis Foundation Medicine, Boehringer INgelheim, and Millennium/Takeda. The authors declare no additional competing financial interests.
Figures




Comment in
-
Prostate cancer: BET inhibitors - SPOP right there!Nat Rev Cancer. 2017 Oct;17(10):574-575. doi: 10.1038/nrc.2017.80. Epub 2017 Sep 8. Nat Rev Cancer. 2017. PMID: 28883513 No abstract available.
-
SPOP tips the balance of BETs in cancer.Nat Med. 2017 Sep 8;23(9):1014-1015. doi: 10.1038/nm.4398. Nat Med. 2017. PMID: 28886001 No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

