Intrinsic BET inhibitor resistance in SPOP-mutated prostate cancer is mediated by BET protein stabilization and AKT-mTORC1 activation
- PMID: 28805822
- PMCID: PMC5653288
- DOI: 10.1038/nm.4379
Intrinsic BET inhibitor resistance in SPOP-mutated prostate cancer is mediated by BET protein stabilization and AKT-mTORC1 activation
Abstract
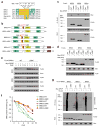
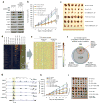
Bromodomain and extraterminal domain (BET) protein inhibitors are emerging as promising anticancer therapies. The gene encoding the E3 ubiquitin ligase substrate-binding adaptor speckle-type POZ protein (SPOP) is the most frequently mutated in primary prostate cancer. Here we demonstrate that wild-type SPOP binds to and induces ubiquitination and proteasomal degradation of BET proteins (BRD2, BRD3 and BRD4) by recognizing a degron motif common among them. In contrast, prostate cancer-associated SPOP mutants show impaired binding to BET proteins, resulting in decreased proteasomal degradation and accumulation of these proteins in prostate cancer cell lines and patient specimens and causing resistance to BET inhibitors. Transcriptome and BRD4 cistrome analyses reveal enhanced expression of the GTPase RAC1 and cholesterol-biosynthesis-associated genes together with activation of AKT-mTORC1 signaling as a consequence of BRD4 stabilization. Our data show that resistance to BET inhibitors in SPOP-mutant prostate cancer can be overcome by combination with AKT inhibitors and further support the evaluation of SPOP mutations as biomarkers to guide BET-inhibitor-oriented therapy in patients with prostate cancer.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Comment in
-
Prostate cancer: BET inhibitors - SPOP right there!Nat Rev Cancer. 2017 Oct;17(10):574-575. doi: 10.1038/nrc.2017.80. Epub 2017 Sep 8. Nat Rev Cancer. 2017. PMID: 28883513 No abstract available.
-
SPOP tips the balance of BETs in cancer.Nat Med. 2017 Sep 8;23(9):1014-1015. doi: 10.1038/nm.4398. Nat Med. 2017. PMID: 28886001 No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

