Mutations in KEOPS-complex genes cause nephrotic syndrome with primary microcephaly
- PMID: 28805828
- PMCID: PMC5819591
- DOI: 10.1038/ng.3933
Mutations in KEOPS-complex genes cause nephrotic syndrome with primary microcephaly
Abstract
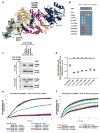
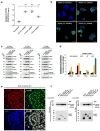
Galloway-Mowat syndrome (GAMOS) is an autosomal-recessive disease characterized by the combination of early-onset nephrotic syndrome (SRNS) and microcephaly with brain anomalies. Here we identified recessive mutations in OSGEP, TP53RK, TPRKB, and LAGE3, genes encoding the four subunits of the KEOPS complex, in 37 individuals from 32 families with GAMOS. CRISPR-Cas9 knockout in zebrafish and mice recapitulated the human phenotype of primary microcephaly and resulted in early lethality. Knockdown of OSGEP, TP53RK, or TPRKB inhibited cell proliferation, which human mutations did not rescue. Furthermore, knockdown of these genes impaired protein translation, caused endoplasmic reticulum stress, activated DNA-damage-response signaling, and ultimately induced apoptosis. Knockdown of OSGEP or TP53RK induced defects in the actin cytoskeleton and decreased the migration rate of human podocytes, an established intermediate phenotype of SRNS. We thus identified four new monogenic causes of GAMOS, describe a link between KEOPS function and human disease, and delineate potential pathogenic mechanisms.
Conflict of interest statement
M.T.C., A.B., and R.E.S. are employees of GeneDx, Gaithersburg, MD, USA.
The other authors declare that they have no competing financial interests.
Figures


Comment in
-
Nephrotic syndrome: Novel monogenic causes of Galloway-Mowat syndrome.Nat Rev Nephrol. 2017 Nov;13(11):661. doi: 10.1038/nrneph.2017.130. Epub 2017 Sep 11. Nat Rev Nephrol. 2017. PMID: 28890537 No abstract available.
References
REFERENCES for Online Methods
References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

