Use of Laboratory Markers in Addition to Symptoms for Diagnosis of Inflammatory Bowel Disease in Children: A Meta-analysis of Individual Patient Data
- PMID: 28806445
- PMCID: PMC5710621
- DOI: 10.1001/jamapediatrics.2017.1736
Use of Laboratory Markers in Addition to Symptoms for Diagnosis of Inflammatory Bowel Disease in Children: A Meta-analysis of Individual Patient Data
Abstract
Importance: Blood markers and fecal calprotectin are used in the diagnostic workup for inflammatory bowel disease (IBD) in pediatric patients. Any added diagnostic value of these laboratory markers remains unclear.
Objective: To determine whether adding laboratory markers to evaluation of signs and symptoms improves accuracy when diagnosing pediatric IBD.
Data sources: A literature search of MEDLINE and EMBASE from inception through September 26, 2016. Studies were identified using indexing terms and free-text words related to child, target condition IBD, and diagnostic accuracy.
Study selection: Two reviewers independently selected studies evaluating the diagnostic accuracy of more than 1 blood marker or fecal calprotectin for IBD, confirmed by endoscopy and histopathology or clinical follow-up, in pediatric patients with chronic gastrointestinal symptoms. Studies that included healthy controls and/or patients with known IBD were excluded.
Data extraction and synthesis: Individual patient data from each eligible study were requested from the authors. In addition, 2 reviewers independently assessed quality with Quality Assessment of Diagnostic Accuracy Studies-2.
Mean outcomes and measures: Laboratory markers were added as a single test to a basic prediction model based on symptoms. Outcome measures were improvement of discrimination by adding markers as a single test and improvement of risk classification of pediatric patients by adding the best marker.
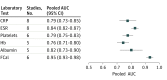
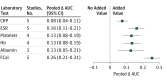
Results: Of the 16 eligible studies, authors of 8 studies (n = 1120 patients) provided their data sets. All blood markers and fecal calprotectin individually significantly improved the discrimination between pediatric patients with and those without IBD, when added to evaluation of symptoms. The best marker-fecal calprotectin-improved the area under the curve of symptoms by 0.26 (95% CI, 0.21-0.31). The second best marker-erythrocyte sedimentation rate-improved the area under the curve of symptoms by 0.16 (95% CI, 0.11-0.21). When fecal calprotectin was added to the model, the proportion of patients without IBD correctly classified as low risk of IBD increased from 33% to 91%. The proportion of patients with IBD incorrectly classified as low risk of IBD decreased from 16% to 9%. The proportion of the total number of patients assigned to the intermediate-risk category decreased from 55% to 6%.
Conclusions and relevance: In a hospital setting, fecal calprotectin added the most diagnostic value to symptoms compared with blood markers. Adding fecal calprotectin to the diagnostic workup of pediatric patients with symptoms suggestive of IBD considerably decreased the number of patients in the group in whom challenges in clinical decision making are most prevalent.
Conflict of interest statement
Figures
References
-
- Levine A, Koletzko S, Turner D, et al. ; European Society of Pediatric Gastroenterology, Hepatology, and Nutrition . ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr. 2014;58(6):795-806. - PubMed
-
- Moons KG, de Groot JA, Linnet K, Reitsma JB, Bossuyt PM. Quantifying the added value of a diagnostic test or marker. Clin Chem. 2012;58(10):1408-1417. - PubMed
-
- Holtman GA, Lisman-van Leeuwen Y, Reitsma JB, Berger MY. Noninvasive tests for inflammatory bowel disease: a meta-analysis. Pediatrics. 2016;137(1):1-11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

