Sema3A Reduces Sprouting of Adult Rod Photoreceptors In Vitro
- PMID: 28806446
- PMCID: PMC5555408
- DOI: 10.1167/iovs.16-21075
Sema3A Reduces Sprouting of Adult Rod Photoreceptors In Vitro
Erratum in
-
Erratum.Invest Ophthalmol Vis Sci. 2017 Sep 1;58(11):4799. doi: 10.1167/iovs.17-22948a. Invest Ophthalmol Vis Sci. 2017. PMID: 28973336 Free PMC article. No abstract available.
Abstract
Purpose: Rod photoreceptor terminals respond to retinal injury with retraction and sprouting. Since the guidance cue Semaphorin3A (Sema3A) is observed in the retina after injury, we asked whether Sema3A contributes to structural plasticity in rod photoreceptors.
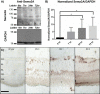
Methods: We used Western blots and alkaline phosphatase (AP)-tagged neuropilin-1 (NPN-1) to detect the expression of Sema3A in an organotypic model of porcine retinal detachment. We then examined Sema3A binding to cultured salamander rod photoreceptors using AP-tagged Sema3A. For functional analysis, we used a microspritzer to apply a gradient of Sema3A-Fc to isolated salamander rod photoreceptors over 24 hours.
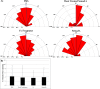
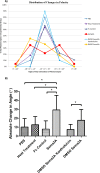
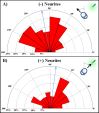
Results: Sema3A protein was biochemically detected in porcine retinal explants in the retina 7, 24, and 72 hours after detachment. In sections, NPN-1 receptor was bound to the inner and outer retina. For isolated rod photoreceptors, Sema3A localized to synaptic terminals and to neuritic processes after 1 week in vitro. In microspritzed rod photoreceptors, process initiation occurred away from high concentrations of Sema3A. Sema3A significantly decreased the number of processes formed by rod photoreceptors although the average length of processes was not affected. The cellular orientation of rod photoreceptors relative to the microspritzer also significantly changed over time; this effect was reduced with the Sema3A inhibitor, xanthofulvin.
Conclusion: Sema3A is expressed in the retina after detachment, binds to rod photoreceptors, affects cell orientation, and reduces photoreceptor process initiation in vitro. Our results suggest that Sema3A contributes to axonal retraction in retinal injury, whereas rod neuritic sprouting and regenerative synaptogenesis may require a reduction in semaphorin signaling.
Figures




References
-
- Marc RE, Jones BW, Watt CB, Strettoi E. . Neural remodeling in retinal degeneration. Prog Retin Eye Res. 2003; 22: 607– 655. - PubMed
-
- Fariss RN, Li Z-Y, Milam AH. . Abnormalities in rod photoreceptors, amacrine cells, and horizontal cells in human retinas with retinitis pigmentosa. Am J Ophthalmol. 2000; 129: 215– 223. - PubMed
-
- Sherry DM, St Jules RS, Townes-Anderson E. . Morphologic and neurochemical target selectivity of regenerating adult photoreceptors in vitro. J Comp Neurol. 1996; 376: 476– 488. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

