Efficacy and safety of sugammadex versus neostigmine in reversing neuromuscular blockade in adults
- PMID: 28806470
- PMCID: PMC6483345
- DOI: 10.1002/14651858.CD012763
Efficacy and safety of sugammadex versus neostigmine in reversing neuromuscular blockade in adults
Abstract
Background: Acetylcholinesterase inhibitors, such as neostigmine, have traditionally been used for reversal of non-depolarizing neuromuscular blocking agents. However, these drugs have significant limitations, such as indirect mechanisms of reversal, limited and unpredictable efficacy, and undesirable autonomic responses. Sugammadex is a selective relaxant-binding agent specifically developed for rapid reversal of non-depolarizing neuromuscular blockade induced by rocuronium. Its potential clinical benefits include fast and predictable reversal of any degree of block, increased patient safety, reduced incidence of residual block on recovery, and more efficient use of healthcare resources.
Objectives: The main objective of this review was to compare the efficacy and safety of sugammadex versus neostigmine in reversing neuromuscular blockade caused by non-depolarizing neuromuscular agents in adults.
Search methods: We searched the following databases on 2 May 2016: Cochrane Central Register of Controlled Trials (CENTRAL); MEDLINE (WebSPIRS Ovid SP), Embase (WebSPIRS Ovid SP), and the clinical trials registries www.controlled-trials.com, clinicaltrials.gov, and www.centerwatch.com. We re-ran the search on 10 May 2017.
Selection criteria: We included randomized controlled trials (RCTs) irrespective of publication status, date of publication, blinding status, outcomes published, or language. We included adults, classified as American Society of Anesthesiologists (ASA) I to IV, who received non-depolarizing neuromuscular blocking agents for an elective in-patient or day-case surgical procedure. We included all trials comparing sugammadex versus neostigmine that reported recovery times or adverse events. We included any dose of sugammadex and neostigmine and any time point of study drug administration.
Data collection and analysis: Two review authors independently screened titles and abstracts to identify trials for eligibility, examined articles for eligibility, abstracted data, assessed the articles, and excluded obviously irrelevant reports. We resolved disagreements by discussion between review authors and further disagreements through consultation with the last review author. We assessed risk of bias in 10 methodological domains using the Cochrane risk of bias tool and examined risk of random error through trial sequential analysis. We used the principles of the GRADE approach to prepare an overall assessment of the quality of evidence. For our primary outcomes (recovery times to train-of-four ratio (TOFR) > 0.9), we presented data as mean differences (MDs) with 95 % confidence intervals (CIs), and for our secondary outcomes (risk of adverse events and risk of serious adverse events), we calculated risk ratios (RRs) with CIs.
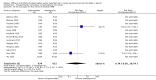
Main results: We included 41 studies (4206 participants) in this updated review, 38 of which were new studies. Twelve trials were eligible for meta-analysis of primary outcomes (n = 949), 28 trials were eligible for meta-analysis of secondary outcomes (n = 2298), and 10 trials (n = 1647) were ineligible for meta-analysis.We compared sugammadex 2 mg/kg and neostigmine 0.05 mg/kg for reversal of rocuronium-induced moderate neuromuscular blockade (NMB). Sugammadex 2 mg/kg was 10.22 minutes (6.6 times) faster then neostigmine 0.05 mg/kg (1.96 vs 12.87 minutes) in reversing NMB from the second twitch (T2) to TOFR > 0.9 (MD 10.22 minutes, 95% CI 8.48 to 11.96; I2 = 84%; 10 studies, n = 835; GRADE: moderate quality).We compared sugammadex 4 mg/kg and neostigmine 0.07 mg/kg for reversal of rocuronium-induced deep NMB. Sugammadex 4 mg/kg was 45.78 minutes (16.8 times) faster then neostigmine 0.07 mg/kg (2.9 vs 48.8 minutes) in reversing NMB from post-tetanic count (PTC) 1 to 5 to TOFR > 0.9 (MD 45.78 minutes, 95% CI 39.41 to 52.15; I2 = 0%; two studies, n = 114; GRADE: low quality).For our secondary outcomes, we compared sugammadex, any dose, and neostigmine, any dose, looking at risk of adverse and serious adverse events. We found significantly fewer composite adverse events in the sugammadex group compared with the neostigmine group (RR 0.60, 95% CI 0.49 to 0.74; I2 = 40%; 28 studies, n = 2298; GRADE: moderate quality). Risk of adverse events was 28% in the neostigmine group and 16% in the sugammadex group, resulting in a number needed to treat for an additional beneficial outcome (NNTB) of 8. When looking at specific adverse events, we noted significantly less risk of bradycardia (RR 0.16, 95% CI 0.07 to 0.34; I2= 0%; 11 studies, n = 1218; NNTB 14; GRADE: moderate quality), postoperative nausea and vomiting (PONV) (RR 0.52, 95% CI 0.28 to 0.97; I2 = 0%; six studies, n = 389; NNTB 16; GRADE: low quality) and overall signs of postoperative residual paralysis (RR 0.40, 95% CI 0.28 to 0.57; I2 = 0%; 15 studies, n = 1474; NNTB 13; GRADE: moderate quality) in the sugammadex group when compared with the neostigmine group. Finally, we found no significant differences between sugammadex and neostigmine regarding risk of serious adverse events (RR 0.54, 95% CI 0.13 to 2.25; I2= 0%; 10 studies, n = 959; GRADE: low quality).Application of trial sequential analysis (TSA) indicates superiority of sugammadex for outcomes such as recovery time from T2 to TOFR > 0.9, adverse events, and overall signs of postoperative residual paralysis.
Authors' conclusions: Review results suggest that in comparison with neostigmine, sugammadex can more rapidly reverse rocuronium-induced neuromuscular block regardless of the depth of the block. Sugammadex 2 mg/kg is 10.22 minutes (˜ 6.6 times) faster in reversing moderate neuromuscular blockade (T2) than neostigmine 0.05 mg/kg (GRADE: moderate quality), and sugammadex 4 mg/kg is 45.78 minutes (˜ 16.8 times) faster in reversing deep neuromuscular blockade (PTC 1 to 5) than neostigmine 0.07 mg/kg (GRADE: low quality). With an NNTB of 8 to avoid an adverse event, sugammadex appears to have a better safety profile than neostigmine. Patients receiving sugammadex had 40% fewer adverse events compared with those given neostigmine. Specifically, risks of bradycardia (RR 0.16, NNTB 14; GRADE: moderate quality), PONV (RR 0.52, NNTB 16; GRADE: low quality), and overall signs of postoperative residual paralysis (RR 0.40, NNTB 13; GRADE: moderate quality) were reduced. Both sugammadex and neostigmine were associated with serious adverse events in less than 1% of patients, and data showed no differences in risk of serious adverse events between groups (RR 0.54; GRADE: low quality).
Conflict of interest statement
Ana‐Marija Hristovska declares no conflict of interest.
Patricia Duch declares no conflict of interest.
Mikkel Allingstrup declares no conflict of interest.
Arash Afshari declares no conflict of interest.
Figures
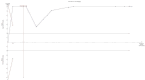
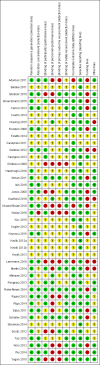
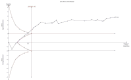
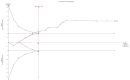

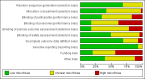





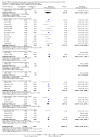
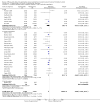

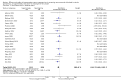



































References
References to studies included in this review
Adamus 2011 {published and unpublished data}
-
- Adamus M, Hrabalek L, Wanek T, Gabrhelik T, Zapletalova J. Intraoperative reversal of neuromuscular block with sugammadex or neostigmine during extreme lateral interbody fusion, a novel technique for spine surgery. Journal of Anesthesia 2011;25(5):716‐20. [PUBMED: 21842171] - PubMed
Balaka 2011 {published data only (unpublished sought but not used)}
-
- Balaka C, Poulida S, Miliatou M, Roussakis A, Lavranou V, Moshaki M, et al. Comparison of sugammadex to neostigmine reversal of neuromuscular blockade in patients with myasthenia gravis. Journal of Cardiothoracic and Vascular Anesthesia. 2011; Vol. 25:S22‐3. [3275631; http://www.jcvaonline.com/article/S1053‐0770(11)00191‐1/pdf]
Blobner 2010 {published and unpublished data}
-
- Blobner M, Eriksson LI, Scholz J, Motsch J, Della Rocca G, Prins ME. Reversal of rocuronium‐induced neuromuscular blockade with sugammadex compared with neostigmine during sevoflurane anaesthesia: results of a randomised, controlled trial. European Journal of Anaesthesiology 2010;27:874‐81. [PUBMED: 20683334] - PubMed
Brueckmann 2015 {published and unpublished data}
-
- Brueckmann B, Sasaki N, Grobara P, Li MK, Woo T, Bie J, et al. Effects of sugammadex on incidence of postoperative residual neuromuscular blockade: a randomized,controlled study. British Journal of Anaesthesia 2015;115(5):743‐51. [PUBMED: 25935840] - PubMed
Carron 2013 {published data only (unpublished sought but not used)}
-
- Carron M, Veronese S, Foletto M, Ori C. Sugammadex allows fast‐track bariatric surgery. Obesity Surgery 2013;23(10):1558‐63. [PUBMED: 23519634] - PubMed
Castro 2014 {published data only (unpublished sought but not used)}
-
- Castro DS Jr, Leão P, Borges S, Gomes L, Pacheco M, Figueiredo P. Sugammadex reduces postoperative pain after laparoscopic bariatric surgery: a randomized trial. Surgical Laparoscopy Endoscopy & Percutaneous Techniques 2014;24(5):420‐3. [PUBMED: 24752165] - PubMed
Cheong 2015 {published data only (unpublished sought but not used)}
Flockton 2008 {published data only (unpublished sought but not used)}
-
- Flockton EA, Mastronardi P, Hunter JM, Gomar C, Mirakhur RK, Aguilera L, et al. Reversal of rocuronium‐induced neuromuscular block with sugammadex is faster than reversal of cisatracurium‐induced block with neostigmine. British Journal of Anaesthesia 2008;100(5):622‐30. [PUBMED: 18385265] - PubMed
Foletto 2014 {published data only (unpublished sought but not used)}
-
- Foletto M, Carron M, Zarantonello F, Prevedello L. Respiratory function after sugammadex and neostigmine administration for reversal of moderate rocuronium‐induced neuromuscular blockade in morbidly obese patients. 8 ed. Springer New York. Montreal, Canada: 19th World Congress of the International Federation for the Surgery of Obesity and Metabolic Disorders, IFSO 2014, 2014; Vol. 24:1139.
Gaszynski 2011 {published data only (unpublished sought but not used)}
-
- Gaszynski T, Szewczyk T, Gaszynski W. Randomized comparison of sugammadex and neostigmine for reversal of rocuronium‐induced muscle relaxation in morbidly obese undergoing general anaesthesia. British Journal of Anaesthesia 2012;108(2):236‐9. [PUBMED: 22012861] - PubMed
Geldner 2012 {published and unpublished data}
-
- Geldner G, Niskanen M, Laurila P, Mizikov V, Hubler M, Beck G, et al. A randomised controlled trial comparing sugammadex and neostigmine at different depths of neuromuscular blockade in patients undergoing laparoscopic surgery. Anaesthesia 2012;67(9):991‐8. [PUBMED: 22698066] - PubMed
Georgiou 2013 {published data only (unpublished sought but not used)}
-
- Georgiou P, Zotou A, Siampalioti A, Tagari P, Tsiotsi P, Filos KS. Clinical and cost‐effectiveness of Sugammadex versus Neostigmine reversal of Rocuronium‐induced neuromuscular block in super obese patients undergoing open laparotomy for bariatric surgery. A randomized controlled trial. European Journal of Anaesthesiology. Barcelona: Euroanesthesia 2013, 2013; Vol. 30:141.
Grintescu 2009 {published data only (unpublished sought but not used)}
-
- Grintescu I, Mirea L, Ologoiu D, Ungureanu R, MekauvarS, Vasilescu M. Comparison of the cost effectiveness of sugammadex and neostigmine during general anaesthesia for laparoscopic cholecystectomy. British Journal of Anaesthesia. 2009; Vol. 103, issue 6:917.
Hakimoglu 2016 {published data only (unpublished sought but not used)}
Illman 2011 {published data only (unpublished sought but not used)}
-
- Illman HL, Laurila P, Antila H, Meretoja OA, Alahuhta S, Olkkola KT. The duration of residual neuromuscular block after administration of neostigmine or sugammadex at two visible twitches during train‐of‐four monitoring. Anesthesia and Analgesia 2011;112(1):63‐8. [PUBMED: 20978247] - PubMed
Isik 2016 {published data only (unpublished sought but not used)}
Jones 2008 {published and unpublished data}
-
- Jones RK, Caldwell JE, Brull SJ, Soto RG. Reversal of profound rocuronium‐induced blockade with sugammadex: a randomized comparison with neostigmine. Anesthesiology 2008;109(5):816–24. [PUBMED: 18946293] - PubMed
Kaufhold 2016 {published data only (unpublished sought but not used)}
-
- Kaufhold N, Schaller SJ, Stäuble CG Baumüller E, Ulm K, Blobner M, et al. Sugammadex and Neostigmine dose‐finding study for reversal of residual neuromuscular block at a train‐of‐four ratio of 0.2 (SUNDRO20). British Journal of Anaesthesia 2016;116(2):233‐40. [PUBMED: 26787792] - PubMed
Khuenl‐Brady 2010 {published data only (unpublished sought but not used)}
-
- Khuenl‐Brady KS, Wattwil M, Vanacker BF, Lora‐Tamayo JI, Rietbergen H, Alvarez‐Gómez JA. Sugammadex provides faster reversal of vecuronium‐induced neuromuscular blockade compared with neostigmine: a multicenter, randomized, controlled trial. Anesthesia and Analgesia 2010;110(1):64‐73. [PUBMED: 19713265] - PubMed
Kizilay 2016 {published and unpublished data}
-
- Kizilay D, Dal D, Saracoglu KT, Eti Z, Gogus FY. Comparison of neostigmine and sugammadex for hemodynamic parameters in cardiac patients undergoing noncardiac surgery. Journal of Clinical Anesthesia 2016;28:30‐5. [PUBMED: 26796612] - PubMed
Koc 2015 {published data only (unpublished sought but not used)}
-
- Koç F, Turan G, Subaşı D, Ekinci O. Comparison of sugammadex and neostigmine in short term surgery [Turkish: Kısa süreli cerrahide sugammadeks ile neostigminin karşılaştırılması]. Journal of Clinical and Analytical Medicine 2015;6(1):41‐5. [3275673; http://www.jcam.com.tr/files/KATD‐1694.pdf]
Kogler 2012 {published data only (unpublished sought but not used)}
-
- KoglerJ, Chalfe N, Karaman Ilić M, Hodoba N. Sugammadex reversal of rocuronium‐induced neuromuscular block in interventional bronchoscopy procedures: a comparison with neostigmine. European Journal of Anaesthesiology. 2012; Vol. 29:146.
Koyuncu 2015 {published data only (unpublished sought but not used)}
-
- Koyuncu O, Turhanoglu S, Ozbakis Akkurt C, Karcıoglu M, Ozkan M, Ozer C, et al. Comparison of sugammadex and conventional reversal on postoperative nausea and vomiting: a randomized, blinded trial. Journal of Clinical Anesthesia 2015;27(1):51‐6. [PUBMED: 25544263] - PubMed
Kvolik 2012a {published data only (unpublished sought but not used)}
-
- Kvolik S, Mraovic B, Kristek J, Stefanic M, Mihaljevic I. Reversal with sugammadex hastens recovery of tracheal reflexes and extubation compared with neostigmine in patients undergoing thyroidectomy. European Journal of Anaesthesiology. 2012; Vol. 29:142.
Kvolik 2012b {published data only (unpublished sought but not used)}
-
- Kvolik S, Mraovic B, Kristek J, Stefanic M, Mihaljevic I. Sugammadex does not influence thyroid hormones in the patients undergoing thyroidectomy in propofol anesthesia. European Journal of Anaesthesiology. 2012; Vol. 29:141.
Kvolik 2013 {published data only (unpublished sought but not used)}
-
- Kvolik S, Rakipovic A, Mraovic B, Kristek J, Ikic V, Kristic M. Faster increase of BIS registered after the reversal of neuromuscular blockade with sugammadex vs. neostigmine. European Journal of Anaesthesiology. 2013; Vol. 30:40.
Lemmens 2010 {published data only (unpublished sought but not used)}
Martini 2014 {published and unpublished data}
-
- Martini CH, Boon M, Bevers RF, Aarts LP, Dahan A. Evaluation of surgical conditions during laparoscopic surgery in patients with moderate vs deep neuromuscular block. British Journal of Anaesthesia 2014;112(3):498‐505. [PUBMED: 24240315] - PubMed
Mekawy 2012 {published data only (unpublished sought but not used)}
-
- Mekawy N, Eman A, Ali F. Improved recovery profiles in sinonasal surgery sugammadex: does it have a role?. Egyptian Journal of Anaesthesia 2012;28(3):175‐8. [DOI: 10.1016/j.egja.2011.12.007] - DOI
Pongracz 2013 {published data only (unpublished sought but not used)}
-
- Pongrácz A, Szatmári S, Nemes R, Fülesdi B, Tassonyi E. Reversal of neuromuscular blockade with sugammadex at the reappearance of four twitches to train‐of‐four stimulation. Anesthesiology 2013;119(1):36‐42. [PUBMED: 23665915] - PubMed
Rahe‐Meyer 2014 {published data only (unpublished sought but not used)}
-
- Rahe‐Meyer N, Fennema H, Schulman S, Klimscha W, Przemeck M, Blobner M, et al. Effect of reversal of neuromuscular blockade with sugammadex versus usual care on bleeding risk in a randomized study of surgical patients. Anesthesiology 2014;121(5):969‐77. [PUBMED: 25208233] - PubMed
Raziel 2013 {published and unpublished data}
-
- Raziel A, Messinger G, Sakran N, Szold A, Goitein D. Comparison of two neuromuscular anesthetics reversal in obese patients undergoing bariatric surgery ‐ a prospective study. 21st International Congress of the European Association for Endoscopic Surgery, EAES. Vienna, Austria, 2013:31.
Riga 2014 {published and unpublished data}
-
- Riga M, Batistaki C, Matsota P, Lyrakos G, Zafeiropoulou F, Kostopanagiotou G. Effect of sugammadex versus neostigmine/atropine combination on cognitive function of adult patients after elective surgery: preliminary results. European Journal of Anaesthesiology. 2014; Vol. 31:157‐8.
Sabo 2011 {published data only (unpublished sought but not used)}
-
- Sabo D, Jones RK, Berry J, Sloan T, Chen JY, Groudine S. Residual neuromuscular blockade at extubation: a randomized comparison of sugammadex and neostigmine reversal of rocuronium‐induced blockade in patients undergoing abdominal surgery. Anesthesia and Clinical Research Journals 2011;2(6):Open assess journal, no pages.
Schaller 2010 {published data only (unpublished sought but not used)}
Sherman 2014 {published data only (unpublished sought but not used)}
-
- Sherman A, Abelansky Y, Evron S, Ezri T. The effect of sugammadex vs. neostigmine on the postoperative respiratory complications following laparoscopic sleeve gastrectomy. European Journal of Anaesthesiology. 2014; Vol. 31:152.
Sustic 2012 {published data only}
-
- Sustic A, Dijana D. Early postoperative gastric emptying in patients undergoing laparoscopic cholecystectomy: sugammadex vs. neostigmine/atropine neuromuscular blockade reversal agents. European Anaesthesiology Congress, EUROANAESTHESIA. Lippincott Williams and Wilkins, 2012; Vol. 29:140.
Tas 2015 {published data only (unpublished sought but not used)}
Woo 2013 {published and unpublished data}
Wu 2014 {published data only (unpublished sought but not used)}
Yagan 2015 {published and unpublished data}
-
- Yagan O, Karakahya RH, Tas N, Canakci E, Hanci V, Yurtlu BS. Intraocular pressure changes associated with tracheal extubation: comparison of sugammadex with conventional reversal of neuromuscular blockade. Journal of the Pakistan Medical Assosication 2015;65(11):1219‐25. [PUBMED: 26564297] - PubMed
References to studies excluded from this review
Aho 2012 {published data only}
-
- Aho Aj, Kamata K, Yli‐Hankala A, Lyytikäinen L‐P, Kulkas A, Jäntti V. Elevated BIS and entropy values after sugammadex or neostigmine: an electroencephalographicor electromyographic phenomenon?. Acta Anaesthesiologica Scandinavica 2012;56(4):465‐73. [PUBMED: 22289106] - PubMed
Baysal 2013 {published data only}
-
- Baysal A, Dogukan M, Toman H, Sagiroglu G, Kocak T. The use of sugammadex for reversal of residual blockade after administration of neostigmine and atropine. European Anaesthesiology Congress, EUROANAESTHESIA. Lippincott Williams and Wilkins, 2013; Vol. 30:142.
Dahaba 2012 {published data only}
-
- Dahaba AA, Bornemann H, Hopfgartner E, Ohran M, Kocher K, Liebmann M, et al. Effect of sugammadex or neostigmine neuromuscular block reversal on bispectral index monitoring of propofol/remifentanil anaesthesia. British Journal of Anaesthesia 2012;108(4):602‐6. [PUBMED: 22315331] - PubMed
Gaona 2012 {published data only}
-
- Gaona D, Carceles MD, Veiga G, Tedesco M, Motta P. Efficacy and safety of the reversal with sugammadex in deep neuromuscular blockade induced by rocuronium in pediatrics. 15th WFSA World Congress of Anaesthesiologists Predio Ferial de Buenos Aires Argentina. Oxford University Press, 2012; Vol. 108:308‐9.
Ghoneim 2015 {published data only}
Harazim 2014 {published data only}
-
- Harazim H, Stourac P, Seidlova D, Adamus M, Krikava I, Pavlik T. Use of rocuronium and active reversal of neuromuscular blockade with sugammadex in anaesthesia for caesarean section led to reduction of myalgia incidence in early postoperative period: prospective randomised interventional multicentric trial. European Journal of Anaesthesiology. 2014; Vol. 31:194‐5.
Kakinuma 2013 {published data only (unpublished sought but not used)}
-
- Kakinuma A, Nagatani H, Yasuda A, Yoshimura T, Sawai J, Nakata Y. Combined use of sugammadex and neostigmine for the reversal of rocuronium‐induced profound neuromuscular blockade. Anesthesia and Clinical Research Journal 2013;4(7):337. [DOI: 10.4172/2155-6148.1000337] - DOI
Kara 2014 {published data only}
-
- Kara T, Ozbagriacik O, Turk HS, Isil CT, Gokuc O, Unsal O, et al. Sugammadex versus neostigmine in pediatric patients: a prospective randomized study. Revista Brasileira de Anestesiologia 2014;64(6):400‐5. [PUBMED: 25437696] - PubMed
Kzlay 2013 {published data only (unpublished sought but not used)}
-
- Kzlay D, Dal D, Saraçoğlu T, Eti Z, Göğüş Y. The effects of sugammadex and neostigmine‐atropine administration on hemodynamic parameters in cardiac patients undergoing non‐cardiac surgery. European Journal of Anaesthesiology. 2013; Vol. 30:75.
Nagashima 2016 {published and unpublished data}
-
- Nagashima S, Takasusuki T, Yamaguchi S, Hamaguchi S. Effects of neostigmine and sugammadex on QT interval and QT dispersion. Dokkyo Journal of Medical Sciences 2016;43:15‐22.
Nagy 2014 {published data only}
-
- Nagy HIA, Elkadi HW. Can sugammadex improve the reversal profile of atracurium under sevoflurane anesthesia?. Egyptian Journal of Anaesthesia 2014;30(1):95‐9. [DOI: 10.1016/j.egja.2013.09.007] - DOI
NCT03111121 {unpublished data only}
-
- NCT03111121. Use of Sugammadex for Reversal of Paralysis in Microlaryngoscopy. https://clinicaltrials.gov/show/NCT03111121, March 23, 2017.
Nemes 2016 {published and unpublished data}
-
- Nemes R, Fülesdi B, Pongrácz A, Asztalos L, Szabó‐Maák Z, Lengyel S, et al. Impact of reversal strategies on the incidence of postoperative residual paralysis after rocuronium relaxation without neuromuscular monitoring: a partially randomised placebo controlled trial. European Journal of Anaesthesiology 2016;1:1. [PUBMED: 28030444] - PubMed
Ozgun 2014 {published data only}
Pecek 2013 {published data only}
-
- Pecek B, Hollan J, Priman T, Tokic Crnic N, Stankovic V, Polh D. New way of dosing sugammadex for termination of vecuronium induced neuromuscular block. Zdravniski Vestnik 2015;84:439–46.
Sacan 2007 {published data only}
-
- Sacan O, White PF, Tufanogullari B, Klein K. Sugammadex reversal of rocuronium‐induced neuromuscular blockade: a comparison with neostigmine‐glycopyrrolate and edrophonium‐atropine. Anesthesia and Analgesia 2007;104(3):569‐74. [PUBMED: 17312210 ] - PubMed
Schepens 2015 {published data only}
-
- Schepens T, Cammu G, Saldien V, Neve N, Jorens PG, Foubert L, et al. Electromyographic activity of the diaphragm during neostigmine or sugammadex‐enhanced recovery after neuromuscular blockade with rocuronium: a randomised controlled study in healthy volunteers. European Journal of Anaesthesiology 2015;32(1):49‐57. [PUBMED: 25111539] - PubMed
Stourac 2016 {published and unpublished data}
-
- Stourac P, Adamus M, Seidlova D, Pavlik T, Janku P, Krikava I, et al. Low‐dose or high‐dose rocuronium reversed with neostigmine or sugammadex for cesarean delivery anesthesia: a randomized controlled noninferiority trial of time to tracheal intubation and extubation. Anesthesia and Analgesia 2016;122(5):1536‐45. [DOI: 10.1213/ANE.0000000000001197; PUBMED: 26974018] - DOI - PubMed
Veiga Ruiz 2011 {published data only}
-
- Veiga Ruiz G, Carceles Baron MD, Dominguez Serrano N, Lopez Fuentes L, Orozco Montes J, Alvarez‐Gomez JA. Sugammadex reversal efficacy and security vs neostigmine in the rocuronium‐induced neuromuscular blockade in paediatric patients. European Journal of Anaesthesiology. Abstracts and Programme: EUROANAESTHESIA 2011: The European Anaesthesiology Congress: Paediatric Anaesthesia and Intensive Care, 2011; Vol. 28:153.
References to studies awaiting assessment
Kim 2016 {published data only}
NCT02243943 {unpublished data only}
-
- NCT02243943. Effect of Neuromuscular Reversal With Sugammadex on Postoperative Recovery Profile (Neuropa). https://clinicaltrials.gov/show/NCT02243943, September 12, 2014.
Sen 2016 {unpublished data only}
-
- Sen A, Erdivanli B, Tomak Y, Pergel A. Reversal of neuromuscular blockade with sugammadex or neostigmine/atropine: effect on postoperative gastrointestinal motility. Journal of Clinical Anesthesia 2016;1:1. [PUBMED: 27290978] - PubMed
References to ongoing studies
NCT01539044 {unpublished data only}
-
- NCT01539044. Optimal Relaxation Technique for Laparotomies With Rocuronium Infusion Followed by Sugammadex Reversal (ProjectO5Rs). https://clinicaltrials.gov/show/NCT01539044, February 15, 2012.
NCT01748643 {unpublished data only}
-
- NCT01748643. CURES: The Effect of Deep Curarisation and Reversal With Sugammadex on Surgical Conditions and Perioperative Morbidity (CURES). https://clinicaltrials.gov/show/NCT01748643, December 6, 2012.
NCT02160223 {unpublished data only}
-
- NCT02160223. Sugammadex Compared With Neostigmin/Atropin for Neuromuscular Block Reversal in Patients With Obstructive Sleep Apnea. https://clinicaltrials.gov/show/NCT02160223, June 8, 2014.
NCT02256280 {unpublished data only}
-
- NCT02256280. A Randomized Double Blind Controlled Trial Comparing Sugammadex and Neostigmine After Thoracic Anesthesia (DATA). https://clinicaltrials.gov/show/NCT02256280, September 24, 2014.
NCT02330172 {unpublished data only}
-
- NCT02330172. Sugammadex Provide Better Surgical Condition Compared With Neostigmine in Laryngeal Microsurgery. https://clinicaltrials.gov/show/NCT02330172, December 9, 2014.
NCT02361060 {unpublished data only}
-
- NCT02361060. Effects of Neuromuscular Block Reversal With Sugammadex vs Neostigmine on Postoperative Respiratory Outcomes After Major Abdominal Surgery. https://clinicaltrials.gov/show/NCT02361060, December 23, 2014.
NCT02414880 {unpublished data only}
-
- NCT02414880. Sugammadex Versus Neostigmine in Patients With Liver Cirrhosis Undergoing Liver Resection. https://clinicaltrials.gov/show/NCT02414880, April 5, 2015.
NCT02454504 {unpublished data only}
-
- NCT02454504. Quality of Awakening and Impact on Cognitive Function After Administration of Sugammadex in Robotic Radical Cystectomy. https://clinicaltrials.gov/show/NCT02454504, May 6, 2015.
NCT02648503 {unpublished data only}
-
- NCT02648503. Deep Neuromuscular Block and Sugammadex Versus Standard of Care on Quality of Recovery in Patients Undergoing Elective Laparoscopic Cholecystectomy. https://clinicaltrials.gov/show/NCT02648503, December 7, 2015.
NCT02666014 {unpublished data only}
-
- NCT02666014. Sugammadex Versus Neostigmine for Postoperative Nausea and Vomiting After Laparoscopic Gynaecological Surgery. https://clinicaltrials.gov/show/NCT02666014, February 2, 2015.
NCT02697929 {unpublished data only}
-
- NCT02697929. Sugammadex/Neostigmine and Liver Transplantation. https://clinicaltrials.gov/show/NCT02697929, February 13, 2016.
NCT02698969 {unpublished data only}
-
- NCT02698969. Recovery of Muscle Function After Deep Neuromuscular Block by Means of Diaphragm Ultrasonography. https://clinicaltrials.gov/show/NCT02698969, February 15, 2016.
NCT02845375 {unpublished data only}
-
- NCT02845375. Effect of Neuromuscular Blockade and Reversal on Breathing (BREATH). https://clinicaltrials.gov/show/NCT02845375, July 14, 2016.
NCT02860507 {unpublished data only}
-
- NCT02860507. Study to Determine if Administration of Sugammadex Impacts Hospital Efficiency. https://clinicaltrials.gov/show/NCT02860507, August 2, 2016.
NCT02861131 {unpublished data only}
-
- NCT02861131. The Effect of Sugammadex Versus Neostigmine on Postoperative Pulmonary Complications. https://clinicaltrials.gov/show/NCT02861131, July 14, 2016.
NCT02909439 {unpublished data only}
-
- NCT02909439. Quality of Recovery After Reversal With Neostigmine or Sugammadex. https://clinicaltrials.gov/show/NCT02909439, August 31, 2016.
NCT02939430 {unpublished data only}
-
- NCT02939430. Sugammadex Reversal of Neuromuscular Blockade and Postoperative Bleeding (Suga_bleeding). https://clinicaltrials.gov/show/NCT02939430, October 17, 2016.
NCT03108989 {unpublished data only}
-
- NCT03108989. Comparison the Postoperative Quality of Recovery Between Neostigmine and Sugammadex in Elderly Patients Undergoing Trans Pars Plana Vitrectomy With General Anesthesia ‐ Randomized Controlled Trial. https://clinicaltrials.gov/show/NCT03108989, April 6, 2017.
NCT03116997 {unpublished data only}
-
- NCT03116997. Study of Recovery of Strength After Surgery Comparing Two Different Medications for Reversal of Muscle Relaxant. https://clinicaltrials.gov/show/NCT03116997, April 12, 2017.
NCT03144453 {unpublished data only}
-
- NCT03144453. Recovery From Anesthesia After Robotic Assisted Radical Cystectomy. https://clinicaltrials.gov/show/NCT03144453, May 3, 2017.
Additional references
Abad‐Gurumeta 2015
-
- Abad‐Gurumeta A, Ripollés‐Melchor J, Casans‐Francés R, Espinosa A, Martínez‐Hurtado E, Fernández‐Pérez C, et al. A systematic review of sugammadex vs neostigmine for reversal of neuromuscular blockade. Anaesthesia 2015;70(12):1441‐52. [PUBMED: 26558858] - PubMed
Agrell 1998
-
- Agrell B, Dehlin O. The clock‐drawing test. Age and Aging 1998;27:399‐403. [PUBMED: 23144287] - PubMed
Ali 1970
-
- Ali HH, Utting JE, Gray C. Stimulus frequency in the detection of neuromuscular block in humans. British Journal of Anaesthesia 1970;42(11):967‐78. [PUBMED: 5488360] - PubMed
Ali 1971
-
- Ali HH, Utting JE, Gray TC. Quantitative assessment of residual antidepolarizing block II. British Journal of Anaesthesia 1971;43(5):478‐85. [PUBMED: 4254032] - PubMed
Altman 2003
Appelbaum 2003
-
- Appelbaum R, Mulder R, Saddler J, Kopman AF, McShane AJ. Atracurium associated with postoperative residual curarization (multiple letters). British Journal of Anaesthesia 2003;90(4):523‐4. - PubMed
Arbous 2005
-
- Arbous MS, Meursing AE, Kleef JW, Lange JJ, Spoormans HH, Touw P, et al. Impact of anaesthesia management characteristics on severe morbidity and mortality. Anesthesiology 2005;102(2):257‐68. [PUBMED: 15681938] - PubMed
Baillard 2005
-
- Baillard C, Clec'h C, Catineau J, Salhi F, Gehan G, Cupa M, et al. Postoperative residual neuromuscular block: a survey of management. British Journal of Anaesthesia 2005;95(5):622‐6. [PUBMED: 16183681 ] - PubMed
Berg 1997
-
- Berg H, Roed J, Viby‐Mogensen J, Mortensen CR, Engbaek J, Skovgaard LT, et al. Residual neuromuscular block is a risk factor for postoperative pulmonary complications. A prospective, randomised, and blinded study of postoperative pulmonary complications after atracurium, vecuronium and pancuronium. Acta Anaesthesiologica Scandinavica 1997;41(9):1095‐103. [PUBMED: 9366929 ] - PubMed
Bevan 1988
-
- Bevan DR, Smith CE, Donati F. Postoperative neuromuscular blockade: a comparison between atracurium, vecuronium and pancuronium. Anesthesiology 1988;69(2):272‐6. [PUBMED: 2900612 ] - PubMed
Bevan 1996
-
- Bevan DR, Kahwaji R, Ansermino JM, Reimer E, Smith MF, O'Connar GA, et al. Residual block after mivacurium with or without edrophonium reversal in adults and children. Anesthesiology 1996;84(2):362‐7. [PUBMED: 8602667 ] - PubMed
Bowman 2006
Bridion 2014
-
- http://www.ema.europa.eu/. Summary of Product Characteristics of BRIDION. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Product_Info....
Brok 2009
-
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive ‐ Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. Journal of Clinical Epidemiology 2009;38(1):287‐98. [PUBMED: 18824466] - PubMed
Brull 1991
-
- Brull SJ, Ehrenwerth J, Connelly NR, Silverman DG. Assessment of residual curarization using low‐current stimulation. Canadian Journal of Anaesthesia 1991;38(2):164‐8. [PUBMED: 1673643 ] - PubMed
Caldwell 2009
-
- Caldwell JE. Clinical limitations of acetylcholinesterase antagonists. Journal of Critical Care 2009;24(1):21‐8. [PUBMED: 19272535] - PubMed
Chan 2004
-
- Chan AW, Hróbjartsson A, Haahr MT, Gøtzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA 2004;291(20):2457‐65. [PUBMED: 15161896] - PubMed
de Boer 2007
-
- Boer HD, Driessen JJ, Marcus MA, Kerkkamp H, Heeringa M, Klimek M. Reversal of rocuronium‐induced (1.2mg/kg) profound neuromuscular block by sugammadex: a multicenter, dose‐finding and safety study. Anesthesiology 2007;107(2):239‐44. [PUBMED: 17667567] - PubMed
De Kam 2014
-
- Kam PJ, Kruithof AC, Lierop MJ, Moerland M, Dennie J, Troyer MD, et al. Lack of a clinically relevant effect of sugammadex on anti‐Xa activity or activated partial thromboplastin time following pretreatment with either unfractionated or low‐molecular‐weight heparin in healthy subjects. International Journal of Clinical Pharmacology and Therapeutics 2014;52(8):631‐41. [PUBMED: 24800921] - PubMed
Debaene 2003
-
- Debaene B, Plaud B, Dilly MP, Donati F. Residual paralysis in the PACU after a single intubating dose of nondepolarizing muscle relaxant with an intermediate duration of action. Anesthesiology 2003;98:1042‐8. [PUBMED: 12717123] - PubMed
Dexter 1995
-
- Dexter F, Coffin S, Tinker JH. Decreases in anesthesia‐controlled time cannot permit one additional surgical operation to be reliably scheduled during the workday. [Decreases in anesthesia‐controlled time cannot permit one additional surgical operation to be reliably scheduled during the workday]. Anesthesia & Analgesia 1995;81(6):1263‐8. [PUBMED: 7486114] - PubMed
Drugs.com
-
- Generic Bridion availability. www.drugs.com. [www.drugs.com/availability/generic‐bridion.html]
Egger 1997
Eikermann 2006
-
- Eikermann M, Blobner M, Groeben H, Rex C, Grote T, Neuhäuser M, et al. Postoperative upper airway obstruction after recovery of the train of four ratio of the adductor pollicis muscle from neuromuscular blockade. Anesthesia and Analgesia 2006;102(3):937‐42. [PUBMED: 16492855] - PubMed
Eriksson 1993
-
- Eriksson LI, Sato M, Severinghaus JW. Effect of vecuronium‐induced partial neuromuscular block on hypoxic ventilatory response. Anesthesiology 1993;78(4):693‐9. [PUBMED: 8096684 ] - PubMed
Eriksson 1997
-
- Eriksson LI, Sundman E, Olsson R, Nilsson L, Witt H, Ekberg O, et al. Functional assessment of the pharynx at rest and during swallowing in partially paralysed humans. Simultaneous video manometry and mechanomyography of awake human volunteers. Anesthesiology 1997;87(5):1035‐43. [PUBMED: 9366453 ] - PubMed
Fawcett 1995
-
- Fawcett WJ, Dash A, Francis GA, Liban JB, Cashman JN. Recovery from neuromuscular blockade: residual curarization following atracurium or vecuronium by bolus dosing or infusions. Acta Anaesthesiologica Scandinavica 1995;39(3):288‐93. [PUBMED: 7793202] - PubMed
Fuchs‐Buder 2012
-
- Fuchs‐Buder T, Meistelman C, Schreiber JU. Is sugammadex economically viable for routine use. Current Opinions in Anesthesiology 2012;25(2):217‐20. [PUBMED: 22157200] - PubMed
Gijsenbergh 2005
-
- Gijsenberg F, Ramael S, Houwing N, Lersel T. First human exposure of Org 25969, a novel agent to reverse the action of rocuronium bromide. Anesthesiology 2007;103(4):695‐703. [PUBMED: 16192761] - PubMed
Golembiewski 2016
-
- Golembiewski J. Sugammadex. Journal of PeriAnesthesia Nursing 2016;31(4):354‐7. [PUBMED: 27444769] - PubMed
GradePro software [Computer program]
-
- gradepro.org. GRADEpro GDT. Version Accessed December 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Hayes 2001
-
- Hayes AH, Mirakyr RK, Brestin DS, Reid JE, McCourt KC. Postoperative residual block after intermediate acting neuromuscular blocking drugs. Anaesthesia 2001;56(4):312‐8. [PUBMED: 11284816 ] - PubMed
Higgins 2011
-
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. handbook.cochrane.org.
Hozo 2005
Hutton 2000
-
- Hutton JL, Williamson PR. Bias in meta‐analysis due to outcome variable selection within studies. Journal of the Royal Statistical Society Series C 2000;49:359‐70. [DOI: 10.1111/1467-9876.00197] - DOI
Insinga 2016
Jensen 1988
-
- Jensen E, Viby‐Mogensen J, Bang U. The Accelograph: a new neuromuscular transmission monitor. Acta Anaesthesiologica Scandinavica 1988;32(1):49‐52. [PUBMED: 2830761] - PubMed
Keating 2016
-
- Keating GM. Sugammadex: a review of neuromuscular blockade reversal. Drugs 2016;76(10):1041‐52. [PUBMED: 27324403] - PubMed
Kim 2002
-
- Kim KS, Lew SH, Cho HY, Cheong MA. Residual paralysis induced by either vecuronium or rocuronium after reversal with pyridostigmine. Anesthesia and Analgesia 2002;95(6):1656‐60. [PUBMED: 12456433 ] - PubMed
Lan 1983
-
- Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika 1983;70(3):659‐63. [DOI: 10.1093/biomet/70.3.659] - DOI
Ledowski 2012
-
- Ledowski T, Hillyard S, Kozman A, Johnston F, Gillies E, Greenaway M, et al. Unrestricted access to sugammadex: impact on neuromuscular blocking agent choice, reversal practice and associated healthcare costs. Anesthesia and Intensive Care Journal 2012;40(2):340‐3. [PUBMED: 22417031] - PubMed
Maybauer 2007
-
- Maybauer DM, Geldner G, Blobner M, Puhringer F, Hofmockel R, Rex C, et al. Incidence and duration of residual paralysis at the end of surgery after multiple administrations of cisatracurium and rocuronium. Anaesthesia 2007;62(1):12‐7. [PUBMED: 17156221] - PubMed
McCaul 2002
-
- McCaul C, Tobin E, Boylan JF, McShane AJ. Atracurium is associated with postoperative residual curarization. British Journal of Anaesthesia 2002;89(5):766‐9. [PUBMED: 12393778] - PubMed
Mirakhur 1985
-
- Mirakhur RK. Antagonism of the muscarinic effects of edrophonium with atropine or glycopyrrolate. A comparative study. British Journal of Anaesthesia 1985;57(12):1213‐6. [PUBMED: 4084429 ] - PubMed
Murphy 2006
-
- Murphy GS. Residual neuromuscular blockade: incidence, assessment, and relevance in the postoperative period. Minerva Anestesiologica 2006;72(3):97‐109. [PUBMED: 16493386] - PubMed
Murphy 2008
-
- Murphy GS, Szokol JW, Marymont JH, Greenberg SB, Avram MJ, Vender JS. Residual neuromuscular blockade and critical respiratory events in the postanesthesia care unit. Anesthesia and Analgesia 2008;107(1):130‐7. [PUBMED: 18635478] - PubMed
Murphy 2010
-
- Murphy GS, Brull SJ. Residual neuromuscular block: lessons unlearned. Part I. Definitions, Incidence, and adverse physiologic effects of residual neuromuscular block. Anesthesia and Analgesia 2010;111(1):120‐8. [PUBMED: 20442260] - PubMed
Murphy 2011
-
- Murphy GS, Szokol JW, Avram MJ, Greenberg SB, Marymont JH, Vender JS, et al. Intraoperative acceleromyography monitoring reduces symptoms of muscle weakness and improves quality of recovery in the early postoperative period. Anesthesiology 2011;115(5):946‐54. [PUBMED: 21946094] - PubMed
Murphy 2013
-
- Murphy GS, Szokol JW, Avram MJ, Greenberg SB, Shear T, Vender JS, et al. Postoperative residual neuromuscular blockade is associated with impaired clinical recovery. Anesthesia and Analgesia 2013;117(1):133‐41. [PUBMED: 23337416] - PubMed
Naguib 2009
-
- Naguib M, Brull SJ. Sugammadex: a novel selective relaxant binding agent. Expert Review of Clinical Pharmacology 2009;2(1):37‐53. [PUBMED: 24422770] - PubMed
Park 2015
Paton 2010
Pedersen 1994
-
- Pedersen T. Complications and death following anaesthesia. A prospective study with special reference to the influence of patient‐, anaesthesia‐, and surgery‐related risk factors. Danish Medical Bulletin 1994;41(3):319‐31. [PUBMED: 7924461] - PubMed
Plaud 2010
-
- Plaud B, Debaene B, Donati F, Marty J. Residual paralysis after emergence from anesthesia. Anesthesiology 2010;112(4):1013‐22. [PUBMED: 20234315] - PubMed
Pogue 1997
-
- Pogue JM, Yusuf S. Cumulating evidence from randomized trials: utilizing sequential monitoring boundaries for cumulative meta‐analysis. Controlled Clinical Trials 1997;18(6):580‐93. [PUBMED: 9408720] - PubMed
Pogue 1998
-
- Pogue J, Yusuf S. Overcoming the limitations of current meta‐analysis of randomised controlled trials. Lancet 1998;351(9095):45‐52. [PUBMED: 9433436] - PubMed
PubChem 2016
-
- National Center for Biotechnology Information. Rocuronium. PubChem Compound Database 2016. [https://pubchem.ncbi.nlm.nih.gov/compound/441290]
Raft 2011
-
- Raft J, Betala Belinga JF, Jurkolow G, Desandes E, Longrois D, Meistelman C. Clinical evaluation of post‐surgical bleeding after a sugammadex injection. Annales Françaises d'Anesthésie et de Réanimation 2011;30(10):714‐7. [PUBMED: 21741200] - PubMed
Reid 2001
-
- Reid JE, Breslin DS, Mirakhur RK, Hayes AH. Neostigmine antagonism of rocuronium block during anesthesia with sevoflurane, isoflurane or propofol. Canadian Journal of Anesthesia 2001;48(4):351‐5. [PUBMED: 11339776] - PubMed
RevMan 5.3.5 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) Version 5.3.5. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2015.
Sauer 2011
-
- Sauer M, Stahn A, Soltesz S, Noeldge‐Schomburg G, Mencke T. The influence of residual neuromuscular block on the incidence of critical respiratory events. A randomised, prospective, placebo‐controlled trial. European Journal of Anaesthesiology 2011;28(12):842‐8. [PUBMED: 21455074] - PubMed
Shorten 1993
-
- Shorten GD. Postoperative residual curarization: incidence, aetiology and associated morbidity. Anaesthesia and Intensive Care 1993;21(6):782‐9. [PUBMED: 8122734 ] - PubMed
Sorgenfrei 2006
-
- Sorgenfrei IF, Norrild K, Larsen PB, Stensballe J, Ostergaard D, Prins ME, et al. Reversal of rocuronium‐induced neuromuscular block by the selective relaxant binding agent sugammadex, a dose‐finding and safety study. Anesthesiology 2006;104(4):667‐74. [PUBMED: 16571960] - PubMed
Sparr 2007
-
- Sparr HJ, Vermeyen KM, Beaufort AM, Rietbergen H, Proost JH, Saldien V, et al. Early reversal of profound rocuronium‐induced neuromuscular blockade by sugammadex in a randomised multicenter study: efficacy, safety, and pharmacokinetics. Anesthesiology 2007;106(5):935‐43. [PUBMED: 17457124 ] - PubMed
Staals 2010
-
- Staals LM, Snoeck MM, Driessen JJ, Hamersvelt HW, Flockton EA, Heuvel MW, et al. Reduced clearance of rocuronium and sugammadex in patients with severe to end‐stage renal failure: a pharmacokinetic study. British Journal of Anaesthesia 2010;104(1):31‐9. [PUBMED: 20007792 ] - PubMed
Sundman 2000
-
- Sundman E, Witt H, Olsson R, Ekberg O, Kuylenstierna R, Eriksson LI. The incidence and mechanisms of pharyngeal and upper oesophageal dysfunction in partially paralysed humans. Pharyngeal video radiography and simultaneous manometry after atracurium. Anesthesiology 2000;92(4):977‐84. [PUBMED: 10754616 ] - PubMed
Thorlund 2009
-
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses?. International Journal of Epidemiology 2009;38(1):276‐86. [PUBMED: 18824467] - PubMed
Tombaugh 1992
-
- Tombaugh TN, McIntyre NJ. The mini‐mental state examination: a comprehensive review. Journal of the American Geriatric Society 1992;40(9):922‐35. [PUBMED: 1512391] - PubMed
Tramer 1999
-
- Tramer MR, Fuchs‐Buder T. Omitting antagonism of neuromuscular block: effect on postoperative nausea and vomiting and risk of residual paralysis. A systematic review. British Journal of Anaesthesia 1999;82(3):379‐86. [PUBMED: 10434820] - PubMed
Viby‐Mogensen 1979
-
- Viby‐Mogensen J, Jørgensen BC, Ording H. Residual curarization in the recovery room. Anesthesiology 1979;50(6):539‐41. [PUBMED: 156513] - PubMed
Viby‐Mogensen 1981
-
- Viby‐Mogensen J, Howardy‐Hansen P, Chraemmer‐Jørgensen B, Ording H, Engbaek J, Nielsen A. Posttetanic count (PTC): a new method of evaluating an intense nondepolarizing neuromuscular blockade. Anesthesiology 1981;55(4):458‐61. [PUBMED: 7294384] - PubMed
Viby‐Mogensen 1988
-
- Viby‐Mogensen J, Jensen E, Werner M, Nielsen HK. Measurement of acceleration: a new method of monitoring neuromuscular function. Acta Anaesthesiologica Scandinavica 1988;32(1):45‐8. [PUBMED: 2830760] - PubMed
Viby‐Mogensen 2000
-
- Viby‐Mogensen J. Postoperative residual curarization and evidence‐based anaesthesia. British Journal of Anaesthesia 2000;84(3):301‐3. [PUBMED: 10793585] - PubMed
Wetterslev 2008
-
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [PUBMED: 18083463] - PubMed
Wetterslev 2009
www.fda.gov
-
- Highligts of prescribing information: BLOXIVERZ™ (neostigmine methylsulfate). www.fda.gov. [https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/204078s000lbl....
References to other published versions of this review
Abrishami 2008
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

