Olaparib protects cardiomyocytes against oxidative stress and improves graft contractility during the early phase after heart transplantation in rats
- PMID: 28806493
- PMCID: PMC5758401
- DOI: 10.1111/bph.13983
Olaparib protects cardiomyocytes against oxidative stress and improves graft contractility during the early phase after heart transplantation in rats
Abstract
Background and purpose: Olaparib, rucaparib and niraparib, potent inhibitors of poly(ADP-ribose) polymerase (PARP) are approved as anti-cancer drugs in humans. Considering the previously demonstrated role of PARP in various forms of acute and chronic myocardial injury, we tested the effects of olaparib in in-vitro models of oxidative stress in cardiomyocytes, and in an in vivo model of cardiac transplantation.
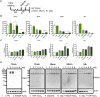
Experimental approach: H9c2-embryonic rat heart-derived myoblasts pretreated with vehicle or olaparib (10μM) were challenged with either hydrogen peroxide (H2 O2 ) or with glucose oxidase (GOx, which generates H2 O2 in the tissue culture medium). Cell viability assays (MTT, lactate dehydrogenase) and Western blotting for PARP and its product, PAR was performed. Heterotopic heart transplantation was performed in Lewis rats; recipients were treated either with vehicle or olaparib (10 mg kg-1 ). Left ventricular function of transplanted hearts was monitored via a Millar catheter. Multiple gene expression in the graft was measured by qPCR.
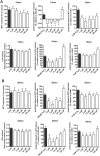
Key results: Olaparib blocked autoPARylation of PARP1 and attenuated the rapid onset of death in H9c2 cells, induced by H2 O2 , but did not affect cell death following chronic, prolonged oxidative stress induced by GOx. In rats, after transplantation, left ventricular systolic and diastolic function were improved by olaparib. In the transplanted hearts, olaparib also reduced gene expression for c-jun, caspase-12, catalase, and NADPH oxidase-2.
Conclusions and implications: Olaparib protected cardiomyocytes against oxidative stress and improved graft contractility in a rat model of heart transplantation. These findings raise the possibility of repurposing this clinically approved oncology drug, to be used in heart transplantation.
Linked articles: This article is part of a themed section on Inventing New Therapies Without Reinventing the Wheel: The Power of Drug Repurposing. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v175.2/issuetoc.
© 2017 The British Pharmacological Society.
Figures






Similar articles
-
The PARP inhibitor olaparib exerts beneficial effects in mice subjected to cecal ligature and puncture and in cells subjected to oxidative stress without impairing DNA integrity: A potential opportunity for repurposing a clinically used oncological drug for the experimental therapy of sepsis.Pharmacol Res. 2019 Jul;145:104263. doi: 10.1016/j.phrs.2019.104263. Epub 2019 May 6. Pharmacol Res. 2019. PMID: 31071432 Free PMC article.
-
The clinically used PARP inhibitor olaparib improves organ function, suppresses inflammatory responses and accelerates wound healing in a murine model of third-degree burn injury.Br J Pharmacol. 2018 Jan;175(2):232-245. doi: 10.1111/bph.13735. Epub 2017 Mar 5. Br J Pharmacol. 2018. PMID: 28146604 Free PMC article.
-
Efficacy of poly (ADP-ribose) polymerase inhibitor olaparib against head and neck cancer cells: Predictions of drug sensitivity based on PAR-p53-NF-κB interactions.Cell Cycle. 2016 Nov 16;15(22):3105-3114. doi: 10.1080/15384101.2016.1235104. Epub 2016 Sep 29. Cell Cycle. 2016. PMID: 27686740 Free PMC article.
-
Poly(ADP-ribose) Polymerase (PARP) and PARP Inhibitors: Mechanisms of Action and Role in Cardiovascular Disorders.Cardiovasc Toxicol. 2018 Dec;18(6):493-506. doi: 10.1007/s12012-018-9462-2. Cardiovasc Toxicol. 2018. PMID: 29968072 Review.
-
Opportunities for the repurposing of PARP inhibitors for the therapy of non-oncological diseases.Br J Pharmacol. 2018 Jan;175(2):192-222. doi: 10.1111/bph.13748. Epub 2017 Mar 26. Br J Pharmacol. 2018. PMID: 28213892 Free PMC article. Review.
Cited by
-
Effects of the Poly(ADP-Ribose) Polymerase Inhibitor Olaparib in Cerulein-Induced Pancreatitis.Shock. 2020 May;53(5):653-665. doi: 10.1097/SHK.0000000000001402. Shock. 2020. PMID: 31274831 Free PMC article.
-
Participation of the ATR/CHK1 pathway in replicative stress targeted therapy of high-grade ovarian cancer.J Hematol Oncol. 2020 Apr 21;13(1):39. doi: 10.1186/s13045-020-00874-6. J Hematol Oncol. 2020. PMID: 32316968 Free PMC article. Review.
-
Naked mole rat cells display more efficient excision repair than mouse cells.Aging (Albany NY). 2018 Jun 20;10(6):1454-1473. doi: 10.18632/aging.101482. Aging (Albany NY). 2018. PMID: 29930219 Free PMC article.
-
Inventing new therapies without reinventing the wheel: the power of drug repurposing.Br J Pharmacol. 2018 Jan;175(2):165-167. doi: 10.1111/bph.14081. Br J Pharmacol. 2018. PMID: 29313889 Free PMC article.
-
The PARP inhibitor olaparib exerts beneficial effects in mice subjected to cecal ligature and puncture and in cells subjected to oxidative stress without impairing DNA integrity: A potential opportunity for repurposing a clinically used oncological drug for the experimental therapy of sepsis.Pharmacol Res. 2019 Jul;145:104263. doi: 10.1016/j.phrs.2019.104263. Epub 2019 May 6. Pharmacol Res. 2019. PMID: 31071432 Free PMC article.
References
-
- Bajaj G, Sharma RK (2006). TNF‐alpha‐mediated cardiomyocyte apoptosis involves caspase‐12 and calpain. Biochem Biophys Res Commun 345: 1558–1564. - PubMed
-
- Berger NA (1985). Poly(ADP‐ribose) in the cellular response to DNA damage. Radiat Res 101: 4–15. - PubMed
-
- Berger NA, Whitacre CM, Hashimoto H, Berger SJ, Chatterjee S (1995). NAD and poly(ADP‐ribose) regulation of proteins involved in response to cellular stress and DNA damage. Biochimie 77: 364–367. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

