Changing the default for tobacco-cessation treatment in an inpatient setting: study protocol of a randomized controlled trial
- PMID: 28806908
- PMCID: PMC5556365
- DOI: 10.1186/s13063-017-2119-9
Changing the default for tobacco-cessation treatment in an inpatient setting: study protocol of a randomized controlled trial
Abstract
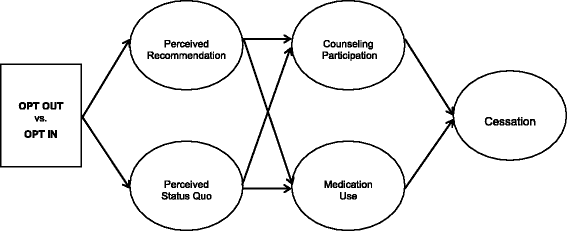
Background: Most health care providers do not treat tobacco dependence routinely. This may in part be due to the treatment "default." Current treatment guidelines recommend that providers (1) ask patients if they are willing to quit and (2) provide cessation-focused medications and counseling only to smokers who state that they are willing to quit. The default is that patients have to "opt in" to receive cessation assistance: providers ask smokers if they are willing to quit, and only offer medications and cessation support to those who say "yes." This drastically limits the reach of cessation services because, at any given encounter, only one in three smokers say that they are ready to quit. The objective of this study is to determine the impact of providing all smokers with tobacco-cessation treatment unless they refuse it (OPT OUT) versus current practice-screening for readiness and only offering treatment to smokers who say they are ready to quit (OPT IN).
Methods: This individually randomized clinical trial is conducted in a tertiary-care hospital. We will conduct the trial among up to 1000 randomly selected hospitalized smokers to determine the population impact of changing the treatment default, identify mediators of outcome, and determine the cost-effectiveness of this new, highly proactive approach. This is a population-based study that targets an endpoint of vital interest; applies minimal eligibility criteria to broaden generalizability; and utilizes hospital staff for interventions to ensure long-term sustainability. The study employs delayed consent and an innovative Bayesian adaptive design to evaluate a major shift in our approach to care. If effective, this change would expand the reach of tobacco-cessation treatment from 30% to 100% of smokers.
Discussion: Regardless of outcome, the trial will provide a model of how to alter and evaluate the impact of health care defaults. If OPT OUT proves to be more effective, it will expand the population eligible for cessation treatment by over 300%. It will also simplify the tobacco-cessation treatment algorithm, and relieve busy health care providers of the burden of evaluating readiness to quit.
Trial registration: Clinical Trials Registration, ID: NCT02721082 . Registered on 22 March 2016.
Keywords: Hospital; Motivation; Randomized clinical trial; Smoking cessation; Tobacco use disorder; Treatment guidelines.
Conflict of interest statement
Authors’ information
BF, EFE, BG, TS, LMM, NN, and KP work for University of Kansas Medical Center and University of Kansas Cancer Center, Kansas City, KS, USA. DC works for Children’s Mercy Hospitals and Clinics, Center for Children’s Healthy Lifestyles and Nutrition, Kansas City, MO, USA. TIS works for Brown University, RI, USA, and TB works for Optum, Seattle, WA, USA.
Ethics approval and consent to participate
The study was approved by the University of Kansas Human Subjects Committee (IRB00006196; STUDY00001774). Consistent with the modified Zelen’s design, consent to participate is received at the 1-month follow-up.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
-
- Henningfield JE, Slade J. Tobacco-dependence medications: public health and regulatory issues. Food Drug Law J. 1998;53(Suppl):75–114. - PubMed
-
- Fiore MC, Jaen CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Clinical practice guideline. Rockville: Department of Health and Human Services. Public Health Service; 2008.
-
- Miller WR, Rollnick S. Motivational interviewing: preparing people for change. 2. New York: Guilford Press; 2002.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical