Tumor microenvironment conditions alter Akt and Na+/H+ exchanger NHE1 expression in endothelial cells more than hypoxia alone: implications for endothelial cell function in cancer
- PMID: 28806945
- PMCID: PMC5556346
- DOI: 10.1186/s12885-017-3532-x
Tumor microenvironment conditions alter Akt and Na+/H+ exchanger NHE1 expression in endothelial cells more than hypoxia alone: implications for endothelial cell function in cancer
Abstract
Background: Chronic angiogenesis is a hallmark of most tumors and takes place in a hostile tumor microenvironment (TME) characterized by hypoxia, low nutrient and glucose levels, elevated lactate and low pH. Despite this, most studies addressing angiogenic signaling use hypoxia as a proxy for tumor conditions. Here, we compared the effects of hypoxia and TME conditions on regulation of the Na+/H+ exchanger NHE1, Ser/Thr kinases Akt1-3, and downstream effectors in endothelial cells.
Methods: Human umbilical vein endothelial cells (HUVEC) and Ea.hy926 endothelial cells were exposed to simulated TME (1% hypoxia, low serum, glucose, pH, high lactate) or 1% hypoxia for 24 or 48 h, with or without NHE1 inhibition or siRNA-mediated knockdown. mRNA and protein levels of NHE1, Akt1-3, and downstream effectors were assessed by qPCR and Western blotting, vascular endothelial growth factor (VEGF) release by ELISA, and motility by scratch assay.
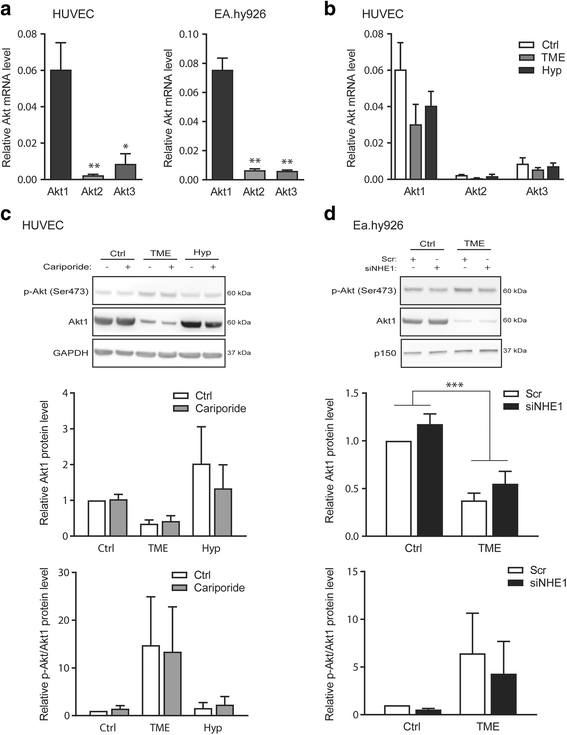
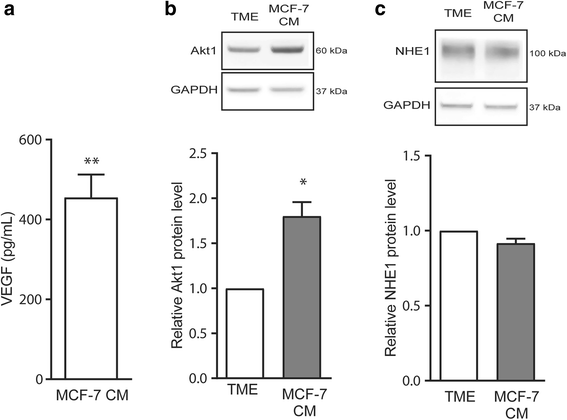
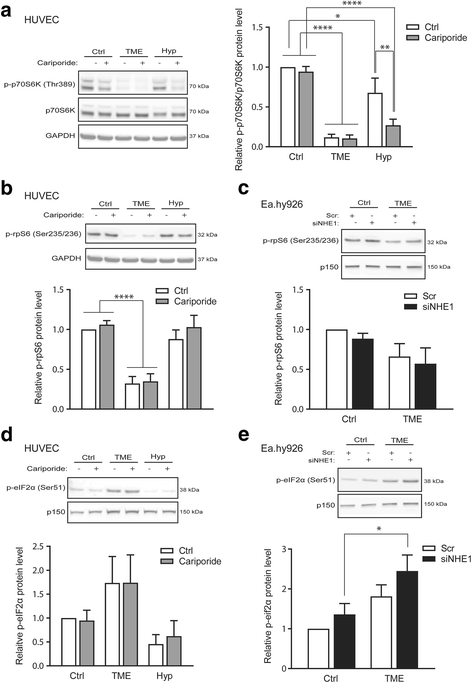
Results: Within 24 h, HIF-1α level and VEGF mRNA level were increased robustly by TME and modestly by hypoxia alone. The NHE1 mRNA level was decreased by both hypoxia and TME, and NHE1 protein was reduced by TME in Ea.hy926 cells. Akt1-3 mRNA was detected in HUVEC and Ea.hy926 cells, Akt1 most abundantly. Akt1 protein expression was reduced by TME yet unaffected by hypoxia, while Akt phosphorylation was increased by TME. The Akt loss was partly reversed by MCF-7 human breast cancer cell conditioned medium, suggesting that in vivo, the cancer cell secretome may compensate for adverse effects of TME on endothelial cells. TME, yet not hypoxia, reduced p70S6 kinase activity and ribosomal protein S6 phosphorylation and increased eIF2α phosphorylation, consistent with inhibition of protein translation. Finally, TME reduced Retinoblastoma protein phosphorylation and induced poly-ADP-ribose polymerase (PARP) cleavage consistent with inhibition of proliferation and induction of apoptosis. NHE1 knockdown, mimicking the effect of TME on NHE1 expression, reduced Ea.hy926 migration. TME effects on HIF-1α, VEGF, Akt, translation, proliferation or apoptosis markers were unaffected by NHE1 knockdown/inhibition.
Conclusions: NHE1 and Akt are downregulated by TME conditions, more potently than by hypoxia alone. This inhibits endothelial cell migration and growth in a manner likely modulated by the cancer cell secretome.
Keywords: Acid–base transport; Angiogenesis; Cancer; Proliferation; Signaling; VEGF; pH regulation.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests. All funding received, including that from the Novo Nordisk Foundation, is independent research funding and does not entail a conflict of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

