Profiling of metalloprotease activities in cerebrospinal fluids of patients with neoplastic meningitis
- PMID: 28806983
- PMCID: PMC5556623
- DOI: 10.1186/s12987-017-0070-5
Profiling of metalloprotease activities in cerebrospinal fluids of patients with neoplastic meningitis
Abstract
Background: Neoplastic invasion into leptomeninges and subarachnoid space, resulting in neoplastic meningitis (NM) is a fatal complication of advanced solid and hematological neoplasms. Identification of malignant involvement of the cerebrospinal fluid (CSF) early in the disease course has crucial prognostic and therapeutic implications, but remains challenging. As indicators of extracellular matrix (ECM) degradation and breakdown of the blood-brain-barrier, Matrix Metalloproteases (MMPs) and A Disintegrin and Metalloproteases (ADAMs) are potential analytes for cerebral pathophysiology and metastatic dissemination of tumor cells into the CSF.
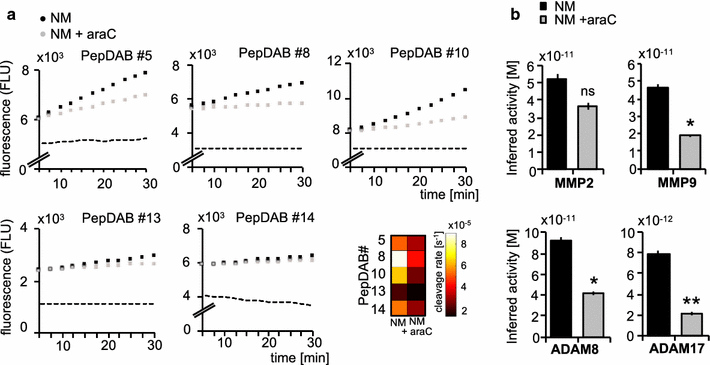
Methods: We compared protease activities in CSF samples from patients with NM and control individuals using FRET-based metalloprotease substrates with distinct enzyme selectivity profiles in a real-time, multiplex approach termed "proteolytic activity matrix assay" (PrAMA). Protease activity dynamics can be tracked by fluorescence changes over time. By simultaneously monitoring a panel of 5 FRET-substrate cleavages, a proteolytic signature can be identified and analyzed to infer the activities of multiple specific proteases. Distinct patterns of substrate cleavage comparing disease vs. control samples allow rapid, reproducible and sensitive discrimination even in small volumes of CSF.
Results: Individual substrate cleavage rates were linked to distinct proteases, and PrAMA computational inference implied increased activities of MMP-9, ADAM8 and ADAM17 (4-5-fold on average) in CSF samples from NM patients that were inhibitable by the metalloprotease inhibitor batimastat (BB-94). The activities of these proteases correlated with blood-brain barrier impairment. Notably, CSF cell counts were not found to directly reflect the protease activities observed in CSF samples from NM patients; this may explain the frequent clinical observation of negative cytology in NM patients.
Conclusion: PrAMA analysis of CSF samples is a potential diagnostic method for sensitive detection of NM and may be suitable for the clinical routine.
Keywords: CSF; FRET-substrates; Metalloproteases; Neoplastic meningitis; Real-time protease activities.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

