A randomized, multi-center, prospective study comparing best medical treatment versus best medical treatment plus renal artery stenting in patients with hemodynamically relevant atherosclerotic renal artery stenosis (RADAR) - one-year results of a pre-maturely terminated study
- PMID: 28807045
- PMCID: PMC5556660
- DOI: 10.1186/s13063-017-2126-x
A randomized, multi-center, prospective study comparing best medical treatment versus best medical treatment plus renal artery stenting in patients with hemodynamically relevant atherosclerotic renal artery stenosis (RADAR) - one-year results of a pre-maturely terminated study
Abstract
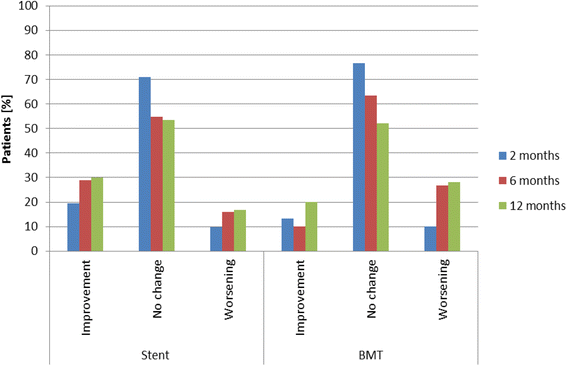
Background: The indications for conservative "best medical treatment" (BMT) versus additional renal artery stenting are a matter of ongoing debate. The RADAR study aimed to evaluate the impact of percutaneous renal artery stenting on the impaired renal function in patients with hemodynamically significant atherosclerotic renal artery stenosis (RAS).
Methods: RADAR is an international, prospective, randomized (1:1) controlled study comparing BMT alone versus BMT plus renal artery stenting in patients with duplex sonographic hemodynamically relevant RAS. Follow-up assessments were at 2, 6, and 12 months and at 3 years. The primary endpoint was change in estimated glomerular filtration rate (eGFR) at 12 months.
Results: Due to slow enrollment, RADAR was terminated early after inclusion of 86 of the scheduled 300 patients (28.7%). Change in eGFR between baseline and 12 months was 4.3 ± 15.4 ml/min/1.73 m2 (stent group) and 3.0 ± 14.9 ml/min/1.73 m2 (BMT group), p > 0.999. Clinical event rates were low with a 12-month composite of cardiac death, stroke, myocardial infarction, and hospitalization for congestive heart failure of 2.9% in the stent and 5.3% in the BMT group, p = 0.526, and a 3-year composite of 14.8% and 12.0%, p = 0.982. At 3 years, target vessel (re-)vascularization occurred in one patient (3.0%) in the stent group and in 8 patients (29.4%) in the BMT group.
Conclusion: In RADAR, outcomes of renal artery stenting were similar to BMT. These results have to be interpreted with the caveat that the study did not reach its statistically based sample size.
Trial registration: Clinicaltrials.gov, NCT00640406. Registered on 17 March 2008.
Keywords: Best medical therapy; Best medical treatment; Hypertension; Renal artery stenosis; Renal artery stenting; Renal function.
Conflict of interest statement
Ethics approval and consent to participate
The study was approved by the respective ethics committees. A respective list is available as a separate file. All patients gave written informed consent.
Consent for publication
Not applicable.
Competing interests
TZ has received consulting fees from Medtronic, Gore, Cook, Bard, Covidien, Biotronik, and Abbott Vascular, and serves on the advisory board of Medtronic, Boston Scientific, and Gore. AE acts as a consultant for and is on the speakers’ bureaus of Abbott Vascular, Biosensors, Biotronik, Boston Scientific, Cordis (Johnson and Johnson), and Medtronic, and has received research contracts from Abbott Vascular and Boston Scientific. DS acts as a member/consultant of the advisory board of Abbott, Biotronik, Boston Scientific, Cook Medical, Cordis, CR Bard, Gardia Medical, and Medtronic/Covidien. BBD and WDY receive consultant fees from Biotronik. YHK, JR, EB, and RW have nothing to disclose.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

