Agonist anti-GITR antibody significantly enhances the therapeutic efficacy of Listeria monocytogenes-based immunotherapy
- PMID: 28807056
- PMCID: PMC5557467
- DOI: 10.1186/s40425-017-0266-x
Agonist anti-GITR antibody significantly enhances the therapeutic efficacy of Listeria monocytogenes-based immunotherapy
Abstract
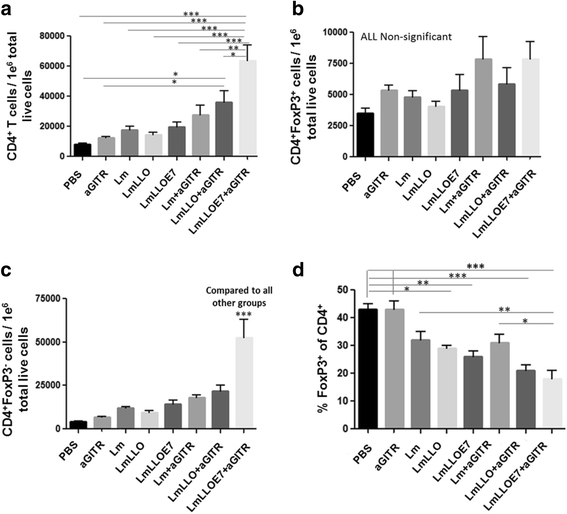
Background: We previously demonstrated that in addition to generating an antigen-specific immune response, Listeria monocytogenes (Lm)-based immunotherapy significantly reduces the ratio of regulatory T cells (Tregs)/CD4+ and myeloid-derived suppressor cells (MDSCs) in the tumor microenvironment. Since Lm-based immunotherapy is able to inhibit the immune suppressive environment, we hypothesized that combining this treatment with agonist antibody to a co-stimulatory receptor that would further boost the effector arm of immunity will result in significant improvement of anti-tumor efficacy of treatment.
Methods: Here we tested the immune and therapeutic efficacy of Listeria-based immunotherapy combination with agonist antibody to glucocorticoid-induced tumor necrosis factor receptor-related protein (GITR) in TC-1 mouse tumor model. We evaluated the potency of combination on tumor growth and survival of treated animals and profiled tumor microenvironment for effector and suppressor cell populations.
Results: We demonstrate that combination of Listeria-based immunotherapy with agonist antibody to GITR synergizes to improve immune and therapeutic efficacy of treatment in a mouse tumor model. We show that this combinational treatment leads to significant inhibition of tumor-growth, prolongs survival and leads to complete regression of established tumors in 60% of treated animals. We determined that this therapeutic benefit of combinational treatment is due to a significant increase in tumor infiltrating effector CD4+ and CD8+ T cells along with a decrease of inhibitory cells.
Conclusion: To our knowledge, this is the first study that exploits Lm-based immunotherapy combined with agonist anti-GITR antibody as a potent treatment strategy that simultaneously targets both the effector and suppressor arms of the immune system, leading to significantly improved anti-tumor efficacy. We believe that our findings depicted in this manuscript provide a promising and translatable strategy that can enhance the overall efficacy of cancer immunotherapy.
Keywords: Anti-GITR antibody; Co-stimulation; Immune tolerance; Immunotherapy; Listeria vaccine; Lm-LLO-E7.
Conflict of interest statement
Ethics approval and consent to participate
All procedures with animals were carried out under the guidelines of the National Institutes of Health and in accordance with approved Augusta University IACUC animal protocol (2011–0399). This study does not involve human participants.
Consent for publication
All authors of the manuscript have read and agreed to its content and are accountable for all aspects of the accuracy and integrity of the manuscript. The manuscript is original, has not already been published, and is not currently under consideration by another journal.
Competing interests
RP is a current employee of Advaxis Inc. which provided Lm, Lm-LLO and Lm-LLO-E7. SK is an Associate Editor for the Journal for ImmunoTherapy of Cancer. Other authors declare no conflict of interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
