An evaluation of progression free survival and overall survival of ovarian cancer patients with clear cell carcinoma versus serous carcinoma treated with platinum therapy: An NRG Oncology/Gynecologic Oncology Group experience
- PMID: 28807367
- PMCID: PMC5697899
- DOI: 10.1016/j.ygyno.2017.08.004
An evaluation of progression free survival and overall survival of ovarian cancer patients with clear cell carcinoma versus serous carcinoma treated with platinum therapy: An NRG Oncology/Gynecologic Oncology Group experience
Abstract
Purpose: We examined disparities in prognosis between patients with ovarian clear cell carcinoma (OCCC) and serous epithelial ovarian cancer (SOC).
Methods: We reviewed data from FIGO stage I-IV epithelial ovarian cancer patients who participated in 12 prospective randomized GOG protocols. Proportional hazards models were used to compare progression-free survival (PFS) and overall survival (OS) by cell type (clear cell versus serous).
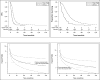
Results: There were 10,803 patients enrolled, 9531 were eligible, evaluable and treated with platinum, of whom 544 (6%) had OCCC, 7054 (74%) had SOC, and 1933 (20%) had other histologies and are not included further. In early stage (I-II) patients, PFS was significantly better in OCCC than in SOC patients. For late stage (III, IV) patients, OCCC had worse PFS and OS compared to SOC, OS HR=1.66 (1.43, 1.91; p<0.001). After adjusting for age and stratifying by protocol and treatment arm, stage, performance status, and race, OCCC had a significantly decreased OS, HR=1.53 (1.33, 1.76; p<0.001). In early stage cases, there was a significantly decreased treatment effect on PFS for consolidative therapy with weekly Paclitaxel versus observation in OCCC compared to SOC (p=0.048).
Conclusions: This is one of the largest analyses to date of OCCC treated on multiple cooperative group trials. OCCC histology is more common than SOC in early stage disease. When adjusted for prognostic factors, in early stage patients, PFS was better for OCCC than for SOC; however, in late-stage patients, OCCC was significantly associated with decreased OS. Finally, treatment effect was influenced by histology.
Keywords: Cancer; Clear cell; Histology; Ovarian; Survival.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
Dr. Robert Mannel wishes to disclose that he has received personal fees from AstraZeneca, Medimmune, Oxigene, Endocyte, Bayer, Clovis and Tesaro outside of the submitted work. Additionally, Dr. Angeles Secord reports grants from AstraZeneca, Elsai, Bristol Myers Squibb, Incyte, Amgen, Genentech, Endocyte, Exelixis, Boerhinger Ingelheim, Astex Pharmaceuticals Inc, Prima Biomed, Abbie-Vie, Astellas Pharma Inc, Tesaro, other from Janssen, Clovis, Genentech, AstraZeneca, Astex, Tesaro, Alexion, outside the submitted work. Finally, Michael Bookman reports grants from AstraZeneca, during the conduct of the study; personal fees from McKesson Specialty Health and USOR, personal fees from Genentech-Roche, personal fees from Mateon, personal fees from AstraZeneca, personal fees from AbbVie, personal fees from Tesaro, personal fees from Endocyte, personal fees from Clovis, personal fees from Pfizer, outside the submitted work. All other authors have nothing to report.
Figures
Comment in
-
Clear Cell Ovarian Cancer: Optimum Management and Prognosis Remain Hazy.Gynecol Oncol. 2017 Nov;147(2):237-239. doi: 10.1016/j.ygyno.2017.10.012. Gynecol Oncol. 2017. PMID: 29096823 No abstract available.
References
-
- Lee KR, Tavassolli FA, Prat J, et al. Surface epithelial stromal tumors. In: Tavassoli FA, Devilee P, editors. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Breast and Female Genital Organs. Lyon: IARC Press; 2003. pp. 117–145.
-
- Kobel M, Kalloger SE, Huntsman DG, et al. Differences in tumor type in low-stage versus high-stage ovarian carcinomas. Int J Gynecol Pathol. 2010;29:203–211. - PubMed
-
- Yahata T, Banzai C, Tanaka K. Histology-specific long-term trends in the incidence of ovarian cancer and borderline tumor in Japanese females: a population-based study from 1983 to 2007 in Niigata. J Obstet Gynaecol Res. 2012;38:645–650. - PubMed
-
- Vasey PA, Jayson GC, Gordon A, et al. Phase III randomized trial of docetaxel-carboplatin versus paclitaxel-carboplatin as first-line chemotherapy for ovarian carcinoma. J Natl Cancer Inst. 2004;96:1682–1691. - PubMed
-
- Sugiyama T, Kamura T, Kigawa J, et al. Clinical characteristics of clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer. 2000;88:2584–2589. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical