Function of Novel Anti-CD19 Chimeric Antigen Receptors with Human Variable Regions Is Affected by Hinge and Transmembrane Domains
- PMID: 28807568
- PMCID: PMC5675490
- DOI: 10.1016/j.ymthe.2017.07.013
Function of Novel Anti-CD19 Chimeric Antigen Receptors with Human Variable Regions Is Affected by Hinge and Transmembrane Domains
Abstract
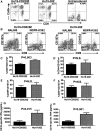
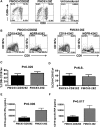
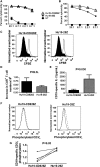
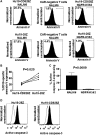
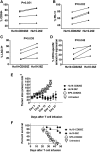
Anti-CD19 chimeric antigen receptor (CAR) T cells have caused remissions of B cell malignancies, but problems including cytokine-mediated toxicity and short persistence of CAR T cells in vivo might limit the effectiveness of anti-CD19 CAR T cells. Anti-CD19 CARs that have been tested clinically had single-chain variable fragments (scFvs) derived from murine antibodies. We have designed and constructed novel anti-CD19 CARs containing a scFv with fully human variable regions. T cells expressing these CARs specifically recognized CD19+ target cells and carried out functions including degranulation, cytokine release, and proliferation. We compared CARs with CD28 costimulatory moieties along with hinge and transmembrane domains from either the human CD28 molecule or the human CD8α molecule. Compared with T cells expressing CARs with CD28 hinge and transmembrane domains, T cells expressing CARs with CD8α hinge and transmembrane domains produced lower levels of cytokines and exhibited lower levels of activation-induced cell death (AICD). Importantly, CARs with hinge and transmembrane regions from either CD8α or CD28 had similar abilities to eliminate established tumors in mice. In anti-CD19 CARs with CD28 costimulatory moieties, lower levels of inflammatory cytokine production and AICD are potential clinical advantages of CD8α hinge and transmembrane domains over CD28 hinge and transmembrane domains.
Keywords: T cell; chimeric antigen receptor; immunotherapy.
Published by Elsevier Inc.
Figures

References
-
- Eshhar Z., Waks T., Gross G., Schindler D.G. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc. Natl. Acad. Sci. U S A. 1993;90:720–724. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

