Thyroid Hormone Receptor-β Agonist GC-1 Inhibits Met-β-Catenin-Driven Hepatocellular Cancer
- PMID: 28807594
- PMCID: PMC5809589
- DOI: 10.1016/j.ajpath.2017.07.005
Thyroid Hormone Receptor-β Agonist GC-1 Inhibits Met-β-Catenin-Driven Hepatocellular Cancer
Abstract
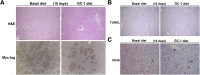
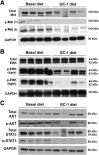
The thyromimetic agent GC-1 induces hepatocyte proliferation via Wnt/β-catenin signaling and may promote regeneration in both acute and chronic liver insufficiencies. However, β-catenin activation due to mutations in CTNNB1 is seen in a subset of hepatocellular carcinomas (HCC). Thus, it is critical to address any effect of GC-1 on HCC growth and development before its use can be advocated to stimulate regeneration in chronic liver diseases. In this study, we first examined the effect of GC-1 on β-catenin-T cell factor 4 activity in HCC cell lines harboring wild-type or mutated-CTNNB1. Next, we assessed the effect of GC-1 on HCC in FVB mice generated by hydrodynamic tail vein injection of hMet-S45Y-β-catenin, using the sleeping beauty transposon-transposase. Four weeks following injection, mice were fed 5 mg/kg GC-1 or basal diet for 10 or 21 days. GC-1 treatment showed no effect on β-catenin-T cell factor 4 activity in HCC cells, irrespective of CTNNB1 mutations. Treatment with GC-1 for 10 or 21 days led to a significant reduction in tumor burden, associated with decreased tumor cell proliferation and dramatic decreases in phospho-(p-)Met (Y1234/1235), p-extracellular signal-related kinase, and p-STAT3 without affecting β-catenin and its downstream targets. GC-1 exerts a notable antitumoral effect on hMet-S45Y-β-catenin HCC by inactivating Met signaling. GC-1 does not promote β-catenin activation in HCC. Thus, GC-1 may be safe for use in inducing regeneration during chronic hepatic insufficiency.
Copyright © 2017 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures









References
-
- Huang Y.H., Tsai M.M., Lin K.H. Thyroid hormone dependent regulation of target genes and their physiological significance. Chang Gung Med J. 2008;31:325–334. - PubMed
-
- Baxter J.D., Webb P. Thyroid hormone mimetics: potential applications in atherosclerosis, obesity and type 2 diabetes. Nat Rev Drug Discov. 2009;8:308–320. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

