Identification and characterization of 5α-cyprinol-sulfating cytosolic sulfotransferases (Sults) in the zebrafish (Danio rerio)
- PMID: 28807679
- PMCID: PMC5675747
- DOI: 10.1016/j.jsbmb.2017.08.005
Identification and characterization of 5α-cyprinol-sulfating cytosolic sulfotransferases (Sults) in the zebrafish (Danio rerio)
Abstract
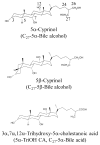
5α-Cyprinol 27-sulfate is the major biliary bile salt present in cypriniform fish including the zebrafish (Danio rerio). The current study was designed to identify the zebrafish cytosolic sulfotransferase (Sult) enzyme(s) capable of sulfating 5α-cyprinol and to characterize the zebrafish 5α-cyprinol-sulfating Sults in comparison with human SULT2A1. Enzymatic assays using zebrafish homogenates showed 5α-cyprinol-sulfating activity. A systematic analysis, using a panel of recombinant zebrafish Sults, revealed two Sult2 subfamily members, Sult2st2 and Sult2st3, as major 5α-cyprinol-sulfating Sults. Both enzymes showed higher activities using 5α-cyprinol as the substrate, compared to their activity with DHEA, a representative substrate for mammalian SULT2 family members, particularly SULT2A1. pH-Dependence and kinetics experiments indicated that the catalytic properties of zebrafish Sult2 family members in mediating the sulfation of 5α-cyprinol were different from those of either zebrafish Sult3st4 or human SULT2A1. Collectively, these results imply that both Sult2st2 and Sult2st3 have evolved to sulfate specifically C27-bile alcohol, 5α-cyprinol, in Cypriniform fish, whereas the enzymatic characteristics of zebrafish Sult3 members, particularly Sult3st4, correlated with those of human SULT2A1.
Keywords: 5α-cyprinol; Cytosolic sulfotransferase; SULT; Sulfation; Zebrafish.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures





References
-
- Alnouti Y. Bile Acid sulfation: a pathway of bile acid elimination and detoxification. Toxicol Sci. 2009;108:225–246. - PubMed
-
- Hofmann AF. Detoxification of lithocholic acid, a toxic bile acid: relevance to drug hepatotoxicity. Drug Metab Rev. 2004;36:703–722. - PubMed
-
- Otterness DM, Wieben ED, Wood TC, Watson RWG, Madden BJ, Mccormick DJ, Weinshilboum RM. Human liver dehydroepiandrosterone sulfotransferase: molecular cloning and expression of cDNA. Mol Pharmacol. 1992;41:865–872. - PubMed
-
- Kitada H, Miyata M, Nakamura T, Tozawa A, Honma W, Shimada M, Nagata K, Sinal CJ, Guo GL, Gonzalez FJ, Yamazoe Y. Protective role of hydroxysteroid sulfotransferase in lithocholic acid-induced liver toxicity. J Biol Chem. 2003;278:17838–17844. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

