Myofibroblast transdifferentiation: The dark force in ocular wound healing and fibrosis
- PMID: 28807717
- PMCID: PMC5600870
- DOI: 10.1016/j.preteyeres.2017.08.001
Myofibroblast transdifferentiation: The dark force in ocular wound healing and fibrosis
Abstract
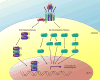
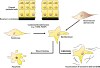
Wound healing is one of the most complex biological processes to occur in life. Repair of tissue following injury involves dynamic interactions between multiple cell types, growth factors, inflammatory mediators and components of the extracellular matrix (ECM). Aberrant and uncontrolled wound healing leads to a non-functional mass of fibrotic tissue. In the eye, fibrotic disease disrupts the normally transparent ocular tissues resulting in irreversible loss of vision. A common feature in fibrotic eye disease is the transdifferentiation of cells into myofibroblasts that can occur through a process known as epithelial-mesenchymal transition (EMT). Myofibroblasts rapidly produce excessive amounts of ECM and exert tractional forces across the ECM, resulting in the distortion of tissue architecture. Transforming growth factor-beta (TGFβ) plays a major role in myofibroblast transdifferentiation and has been implicated in numerous fibrotic eye diseases including corneal opacification, pterygium, anterior subcapsular cataract, posterior capsular opacification, proliferative vitreoretinopathy, fibrovascular membrane formation associated with proliferative diabetic retinopathy, submacular fibrosis, glaucoma and orbital fibrosis. This review serves to introduce the pathological functions of the myofibroblast in fibrotic eye disease. We also highlight recent developments in elucidating the multiple signaling pathways involved in fibrogenesis that may be exploited in the development of novel anti-fibrotic therapies to reduce ocular morbidity due to scarring.
Keywords: Epithelial-mesenchymal transition (EMT); Fibrosis; Myofibroblast; Ocular; Transforming growth factor-beta (TGFβ); Wound healing.
Crown Copyright © 2017. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors have no financial disclosures or conflicts of interest.
Figures


References
-
- Abu El-Asrar AM, Struyf S, Kangave D, Geboes K, Van Damme J. Chemokines in proliferative diabetic retinopathy and proliferative vitreoretinopathy. Eur. Cytokine. Netw. 2006;17:155–165. - PubMed
-
- Abu El-Asrar AM, van den Steen PE, Al-Amro SA, Missotten L, Opdenakker G, Geboes K. Expression of angiogenic and fibrogenic factors in proliferative vitreoretinal disorders. Int. Ophthalmol. 2007;27:11–22. - PubMed
-
- Abu El-Asrar AM, De Hertogh G, van den Eynde K, Alam K, van Raemdonck K, Opdenakker G, van Damme J, Geboes K, Struyf S. Myofibroblasts in proliferative diabetic retinopathy can originate from infiltrating fibrocytes and through endothelial-to-mesenchymal transition (EndoMT) Exp. Eye. Res. 2015;132:179–189. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

