Adolescent binge ethanol exposure alters specific forebrain cholinergic cell populations and leads to selective functional deficits in the prefrontal cortex
- PMID: 28807788
- PMCID: PMC5609857
- DOI: 10.1016/j.neuroscience.2017.08.013
Adolescent binge ethanol exposure alters specific forebrain cholinergic cell populations and leads to selective functional deficits in the prefrontal cortex
Erratum in
-
Corrigendum to "Adolescent Binge Ethanol Exposure Alters Specific Forebrain Cholinergic Cell Populations and Leads to Selective Functional Deficits in the Prefrontal Cortex" [Neuroscience 361 (2017) 129-143].Neuroscience. 2018 Apr 1;375:169. doi: 10.1016/j.neuroscience.2018.02.011. Epub 2018 Feb 21. Neuroscience. 2018. PMID: 29474829 Free PMC article. No abstract available.
Abstract
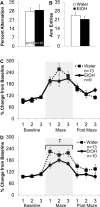
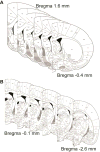
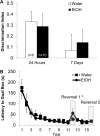
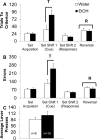
Adolescence has been identified as a vulnerable developmental time period during which exposure to drugs can have long-lasting, detrimental effects. Although adolescent binge-like ethanol (EtOH) exposure leads to a significant reduction in forebrain cholinergic neurons, EtOH's functional effect on acetylcholine (ACh) release during behavior has yet to be examined. Using an adolescent intermittent ethanol exposure model (AIE), rats were exposed to binge-like levels of EtOH from postnatal days (PD) 25 to 55. Three weeks following the final EtOH exposure, cholinergic functioning was assessed during a spontaneous alternation protocol. During maze testing, ACh levels increased in both the hippocampus and prefrontal cortex. However, selectively in the prefrontal cortex, AIE rats displayed reduced levels of behaviorally relevant ACh efflux. We found no treatment differences in spatial exploration, spatial learning, spatial reversal, or novel object recognition. In contrast, AIE rats were impaired during the first attentional set shift on an operant set-shifting task, indicative of an EtOH-mediated deficit in cognitive flexibility. A unique pattern of cholinergic cell loss was observed in the basal forebrain following AIE: Within the medial septum/diagonal band there was a selective loss (30%) of choline acetyltransferase (ChAT)-positive neurons that were nestin negative (ChAT+/nestin-); whereas in the Nucleus basalis of Meynert (NbM) there was a selective reduction (50%) in ChAT+/nestin+. These results indicate that early adolescent binge EtOH exposure leads to a long-lasting frontocortical functional cholinergic deficit, driven by a loss of ChAT+/nestin+ neurons in the NbM, which was associated with impaired cognitive flexibility during adulthood.
Copyright © 2017 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures




Similar articles
-
Dysregulation of neurotrophin expression in prefrontal cortex and nucleus basalis magnocellularis during and after adolescent intermittent ethanol exposure.Alcohol. 2024 Nov;120:1-14. doi: 10.1016/j.alcohol.2024.06.001. Epub 2024 Jun 17. Alcohol. 2024. PMID: 38897258
-
Voluntary wheel running exercise rescues behaviorally-evoked acetylcholine efflux in the medial prefrontal cortex and epigenetic changes in ChAT genes following adolescent intermittent ethanol exposure.PLoS One. 2024 Oct 22;19(10):e0311405. doi: 10.1371/journal.pone.0311405. eCollection 2024. PLoS One. 2024. PMID: 39436939 Free PMC article.
-
Neuroimmune and epigenetic involvement in adolescent binge ethanol-induced loss of basal forebrain cholinergic neurons: Restoration with voluntary exercise.Addict Biol. 2020 Mar;25(2):e12731. doi: 10.1111/adb.12731. Epub 2019 Feb 18. Addict Biol. 2020. PMID: 30779268 Free PMC article.
-
Organization of the basal forebrain cholinergic system in the human brain.Handb Clin Neurol. 2025;211:11-21. doi: 10.1016/B978-0-443-19088-9.00009-3. Handb Clin Neurol. 2025. PMID: 40340056 Review.
-
Cholinergic circuits in cognitive flexibility.Neuroscience. 2017 Mar 14;345:130-141. doi: 10.1016/j.neuroscience.2016.09.013. Epub 2016 Sep 15. Neuroscience. 2017. PMID: 27641830 Review.
Cited by
-
Potential Role of Extracellular CIRP in Alcohol-Induced Alzheimer's Disease.Mol Neurobiol. 2020 Dec;57(12):5000-5010. doi: 10.1007/s12035-020-02075-1. Epub 2020 Aug 21. Mol Neurobiol. 2020. PMID: 32827106 Free PMC article. Review.
-
Relationship of Wine Consumption with Alzheimer's Disease.Nutrients. 2020 Jan 13;12(1):206. doi: 10.3390/nu12010206. Nutrients. 2020. PMID: 31941117 Free PMC article. Review.
-
Effect of alcohol use on the adolescent brain and behavior.Pharmacol Biochem Behav. 2020 May;192:172906. doi: 10.1016/j.pbb.2020.172906. Epub 2020 Mar 13. Pharmacol Biochem Behav. 2020. PMID: 32179028 Free PMC article. Review.
-
Adolescent binge alcohol exposure accelerates Alzheimer's disease-associated basal forebrain neuropathology through proinflammatory HMGB1 signaling.Front Aging Neurosci. 2025 Feb 19;17:1531628. doi: 10.3389/fnagi.2025.1531628. eCollection 2025. Front Aging Neurosci. 2025. PMID: 40046779 Free PMC article.
-
The Effect of Chronic Alcohol on Cognitive Decline: Do Variations in Methodology Impact Study Outcome? An Overview of Research From the Past 5 Years.Front Neurosci. 2022 Mar 10;16:836827. doi: 10.3389/fnins.2022.836827. eCollection 2022. Front Neurosci. 2022. PMID: 35360176 Free PMC article. Review.
References
-
- Allison C, Shoaib M. Nicotine improves performance in an attentional set shifting task in rats. Neuropharm. 2013;64:314–320. - PubMed
-
- Beaudet G, Valable S, Bourgine J, Lelong-Boulouard V, Lanfumey L, Freret T, Boulouard M, Paizanis E. Long-Lasting Effects of Chronic Intermittent Alcohol Exposure in Adolescent Mice on Object Recognition and Hippocampal Neuronal Activity. Alcohol. Clin. Exp. Res. 2016;40:2591–2603. - PubMed
-
- Becker N, Kalpouzos G, Persson J, Laukka EJ, Brehmer Y. Differential Effects of Encoding Instructions on Brain Activity Patterns of Item and Associative Memory. J. Cogn. Neurosci. 2017;29:545–559. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

