Cortical representation of persistent visual stimuli
- PMID: 28807872
- PMCID: PMC5957542
- DOI: 10.1016/j.neuroimage.2017.08.028
Cortical representation of persistent visual stimuli
Abstract
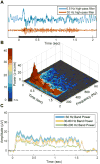
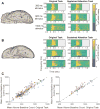
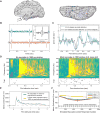
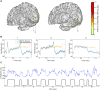
Research into visual neural activity has focused almost exclusively on onset- or change-driven responses and little is known about how information is encoded in the brain during sustained periods of visual perception. We used intracranial recordings in humans to determine the degree to which the presence of a visual stimulus is persistently encoded by neural activity. The correspondence between stimulus duration and neural response duration was strongest in early visual cortex and gradually diminished along the visual hierarchy, such that is was weakest in inferior-temporal category-selective regions. A similar posterior-anterior gradient was found within inferior temporal face-selective regions, with posterior but not anterior sites showing persistent face-selective activity. The results suggest that regions that appear uniform in terms of their category selectivity are dissociated by how they temporally represent a stimulus in support of ongoing visual perception, and delineate a large-scale organizing principle of the ventral visual stream.
Keywords: Early visual cortex; Electrocorticography; Fusiform face area; High-frequency activity; Inferior temporal cortex; Sustained perception; Visual cortex.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





References
-
- Allison T, Puce A, Spencer DD, McCarthy G, Belger A. Electrophysiological studies of human face perception. I: potential generated in occiptotemporal cortex by face and non-face stimuli. Cereb Cortex. 1999;9:415–430. - PubMed
-
- Argall BD, Saad ZS, Beauchamp MS. Simplified intersubject averaging on the cortical surface using SUMA. Hum Brain Mapp. 2006;27:14–27. http://dx.doi.org/10.1002/hbm.20158. - DOI - PMC - PubMed
-
- Avidan G, Harel M, Hendler T, Benbashat D, Zohary E, Papanikolaou A, Keliris Ga, Papageorgiou TD, Shao Y, Krapp E, Papageorgiou E, Stingl K, Bruckmann A, Schiefer U, Logothetis NK, Smirnakis SM. Contrast sensitivity in human visual areas and its relationship to object recognition. J Neurophysiol. 2002;87:3102–3116. - PubMed
-
- Benjamini Y, Yosef H. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. 1995;57:289–300.
-
- Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological studies of face perception in humans. J Cogn Neurosci. 1996;8:551–565. http://dx.doi.org/10.1162/jocn.1996.8.6.551. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

